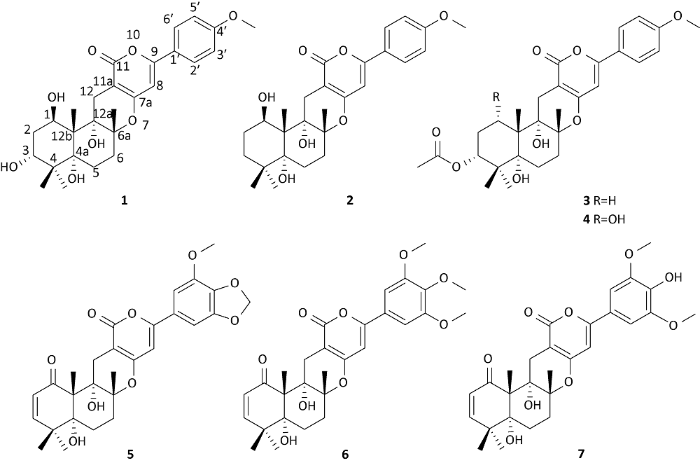
红树林是生长在热带、亚热带海洋潮间带的耐盐植物群落,其独特的生态环境养育了大量具有特色的微生物类群,进而产生了大量结构新颖、生物活性多样的次生代谢产物(Debbab et al. 2013;Wu et al. 2019)。在前期的研究中,本课题组从泰国红树来源的拟茎点霉属内生真菌中获得了一系列结构新颖的化合物(Luo et al. 2016;Wang et al. 2017;Hu et al. 2018)。曲霉属真菌广泛分布于空气、土壤及食物中,其次生代谢产物是天然药物的重要来源。近年来,从土曲霉Aspergillus terreus中分离鉴定的化合物主要有洛伐他丁、丁烯酸内酯、生物碱和杂萜类化合物(Cai et al. 2013;Matsuda et al. 2015;Guo et al. 2016;Qi et al. 2016),另外,还有少量的二倍半萜、聚酮和甾体等化合物的报道(Elkhayat et al. 2016;Liu et al. 2016;Xu et al. 2017)。其中,杂萜是土曲霉次生代谢产物中结构最为复杂的一类化合物(齐昌兴 2017),具有抑制乙酰胆碱酯酶(AchE)、抗病毒和抗炎等多种生物活性(Yoo et al. 2005;Nong et al. 2014;Liaw et al. 2015)。本研究对一株来源于红树植物木果楝中的内生真菌Aspergillus terreus xy03进行发酵培养,综合运用多种色谱技术、波谱技术从中分离鉴定了7个杂萜类化合物(图1),包括1个新化合物。
图1
1 材料与方法
1.1 材料
1.1.1 菌株:Aspergillus terreus xy03于2012年8月分离自泰国南部董里府海岸的马六甲木果楝Xylocarpus moluccensis中,由中国医学科学院北京协和医学院药物研究所戴均贵研究员鉴定为土曲霉Aspergillus terreus(李宁等 2013),标本保存于暨南大学海洋药物研究中心。
1.1.2 实验仪器和试剂:Bruker AV-400型核磁共振波谱仪(德国Bruker公司);Waters 2535型高效液相色谱仪(美国Waters公司);MCP 200型旋光测定仪(德国Anton Paar公司);Agilent 6210 LC/MSD TOF型质谱仪(美国Agilent公司);Hei-VAP advantage型旋转蒸发仪(德国Heidolph公司);GENESYS 10S紫外-可见分光光度仪(美国Thermofisher公司);DLSB-5/25低温冷却循环泵(上海霄汉实业发展有限公司);全温度振荡培养箱(太仓市华美生化仪器厂);EnSpire 2300酶标仪(美国PerkinElmer公司);GF254薄层色谱硅胶预制板(烟台化学工业研究所);正相柱层析硅胶(青岛海洋化工有限公司);反相柱层析硅胶(日本YMC公司);Sephadex LH-20(美国GE公司);YMC C18色谱柱(250mm× 10mm,5μm);电鳐乙酰胆碱酯酶(AChE)、5,5-硫代-双-2-硝基苯甲酸(DTNB)、碘化硫代乙酰胆碱(ATCh)和阳性对照石杉碱甲(Hup.A)均购自美国Sigma公司;氘代试剂(美国CIL公司);色谱甲醇、乙腈(德国Merck公司);常用有机试剂均为天津大茂产分析纯。
1.2 菌株发酵
将保藏于4℃冰箱的菌种接种到PDA平板,28℃培养箱中培养3d后,挑取菌丝接种到2瓶装有250mL PDB的锥形瓶中,28℃恒温振荡(120r/min)培养3d作为种子液。将种子液接种至大米固体培养基中(每瓶50g大米,70mL 3%盐水,120℃高压灭菌,共80瓶),室温下静置培养69d。
1.3 提取和分离
将培养基和菌体进行机械粉碎,甲醇浸泡提取3次,合并提取液,减压浓缩得总浸膏。总浸膏加水混悬,用3倍量的乙酸乙酯萃取3次,得到乙酸乙酯部位约300g。乙酸乙酯部位经硅胶柱层析(氯仿-甲醇10:0-1:1)梯度洗脱,运用TLC和HPLC分析合并后得到10个主流分(Fr.1-Fr.10)。其中Fr.2经反相柱层析(甲醇-水1:9-10:0)、Sephadex LH-20(氯仿-甲醇1:1)以及半制备HPLC(乙腈-水50:50)纯化得到化合物5(4.5mg,tR 16min)、6(5.8mg,tR 24min)和7(3.6mg,tR 12min)。Fr.3经反相柱层析(甲醇-水1:9-10:0)、凝胶Sephadex LH-20(甲醇洗脱)得到Fr.3.2,再经半制备HPLC(乙腈-水50:50)纯化得到化合物3(6mg,tR 46min)和4(2mg,tR 36min)。Fr.7经反相柱层析(甲醇-水1:9-10:0)、凝胶Sephadex LH-20(甲醇洗脱)得到Fr.7.6,然后通过半制备HPLC(乙腈-水47:53)纯化得到化合物1(7.4mg,tR 11min)和2(3.9mg,tR 9min)。
1.4 乙酰胆碱酯酶抑制活性测试
采用改进的Ellman法(Ellman et al. 1961)对化合物1-7进行乙酰胆碱酯酶抑制活性测试。取40μL 0.2mmol/L样品溶液、20μL 0.2U/mL AChE和20μL 3mmol/L ATCh于96孔板中。在37℃下条件下孵育30min后置于冰浴中30s以上终止反应,并加入10μL 1mmol/L HCl和120μL 0.7mmol/L DTNB显色,5min内用酶标仪检测各孔在405nm处的OD值。以石杉碱甲(Hup.A,0.1mmol/L)为阳性对照,实验重复3次。单体化合物对AChE的抑制率I(%)计算公式如下:
$I=\frac{OD_{对照组}-OD_{实验组}}{OD_{对照组}}$
2 结果与分析
2.1 结构鉴定
化合物1:白色针状晶体(甲醇);[α]+26(c 0.1,CH3OH);UV(CH3OH)λmax (log ε):219(3.96),253(4.10),330(4.19)nm;1H,13C NMR和HMBC数据见表1;HR-ESI-MS m/z 487.2328 [M+H]+(calcd for C27H35O8,487.2326),不饱和度为11。1H NMR(400MHz, CD3OD)(表1)显示有30个质子信号,包括1个1,4-二取代苯环质子信号[δH 7.79(2H, d, J=9.0Hz, H-2’/H-6’),7.02(2H, d, J=9.0Hz, H-3’/H-5’)],1个烯氢质子信号[δH 6.54(1H, s, H-8)],2个连氧次甲基质子信号[δH 4.82(1H, dd, J=11.5, 5.0Hz, H-1),3.59(1H, dd, J=3.2, 2.8Hz, H-3)],4个亚甲基质子信号[δH 3.16(1H, d, J=17.9Hz, H-12α),2.83(1H, d, J=17.9Hz, H-12β),2.38(1H, td, J=13.6, 4.3Hz, H-6α),2.15(1H, m, H-2β),2.01(1H, m, H-2α),1.93(1H, dd, J=14.0, 3.9Hz, H-5β),1.78(1H, m, H-5α),1.69(1H, m, H-6β)],1个甲氧基质子信号[δH 3.85(3H, s, 4’-OCH3)]和4个甲基质子信号[δH 1.47(3H, s, 6a-CH3),1.19(3H, s, 12b-CH3),1.10(3H, s, 4α-CH3),1.04(3H, s, 4β-CH3)]。13C NMR(100MHz,CD3OD)(表1)显示有27个碳信号,包括1个酯羰基碳信号(δC 167.3),10个烯碳信号(δC 165.6, 163.2, 159.6, 128.1, 128.1, 125.1, 115.4, 115.4, 99.3, 98.2),5个季碳信号(δC 85.4, 83.2, 78.7, 49.4, 42.5),2个连氧次甲基碳信号(δC 79.0, 66.1),1个甲氧基碳信号(δC 55.9),4个亚甲基碳信号(δC 37.0, 30.0, 29.7, 26.6)以及4个甲基碳信号(δC 24.8, 24.6, 23.7, 17.5)。
表1 化合物1的NMR数据(400MHz,CD3OD)
Table 1
| 位置 Position | δH | δC | HMBC(H→ C#) |
|---|---|---|---|
| 1 | 4.82, dd (11.5, 5.0) | 66.1, CH | 2, 12a, 12b, 12b-CH3 |
| 2 | β 2.15, m | 37.0, CH2 | 1, 4, 12b |
| α 2.01, m | 1, 4, 12b | ||
| 3 | 3.59, dd (3.2, 2.8) | 79.0, CH | 1, 2, 4, 4a, 4α-CH3, 4β-CH3 |
| 4 | 42.5, qC | ||
| 4a | 85.4, qC | ||
| 5 | β 1.93, dd (14.0, 3.9) | 26.6, CH2 | 4, 4a, 6a, 12b |
| α 1.78, m | 4, 4a, 6a, 12b | ||
| 6 | α 2.38, td (13.6, 4.3) | 30.0, CH2 | 6a, 6α-CH3 |
| β 1.69, m | 6a, 6α-CH3 | ||
| 6a | 83.2, qC | ||
| 7a | 165.6, qC | ||
| 8 | 6.54, s | 98.2, CH | 7a, 9, 11a, 1’ |
| 9 | 159.6, qC | ||
| 11 | 167.3, qC | ||
| 11a | 99.3, qC | ||
| 12 | α 3.16, d (17.9) | 29.7, CH2 | 6a, 6a-CH3, 7a, 11a, 11, 12a, 12b |
| β 2.83, d (17.9) | 6a, 6a-CH3, 7a, 11a, 11, 12a, 12b | ||
| 12a | 78.7, qC | ||
| 12b | 49.4, qC | ||
| 4α-CH3 | 1.10, s | 23.7, CH3 | 3, 4, 4a |
| 4β-CH3 | 1.04, s | 24.6, CH3 | 3, 4, 4a |
| 6a-CH3 | 1.47, s | 24.8, CH3 | 6, 6a, 12a |
| 12b-CH3 | 1.19, s | 17.5, CH3 | 1, 4a, 12a, 12b |
| 1’ | 125.1, qC | ||
| 2’ | 7.79, d (9.0) | 128.1, CH | 9, 1’, 4’ |
| 3’ | 7.02, d (9.0) | 115.4, CH | 1’, 4’ |
| 4’ | 163.2, qC | ||
| 5’ | 7.02, d (9.0) | 115.4, CH | 1’, 4’ |
| 6’ | 7.79, d (9.0) | 128.1, CH | 9, 1’, 4’ |
| 4’-OCH3 | 3.85, s | 55.9, CH3 | 4’ |
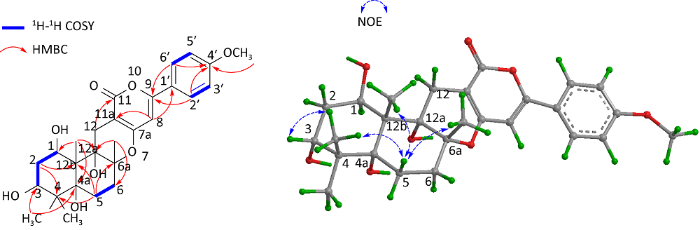
化合物1的核磁数据与(1R,4aR,6aR, 12aR,12bS)-1,3,4,4a,5,6,6a,12,12a,12b-decahydro-1,4a,12a-trihydroxy-4,4,6a,12b-tetramethyl-9-(4’-methoxyphenyl)-2H,11H-naphtho[2,1-b]pyrano[3,4-e]pyran-11-one(2)非常相似,主要区别在于化合物1比化合物2少1个亚甲基信号,多1个连氧次甲基信号[δH 3.59 (1H, dd)/δC 79.0]。在HMBC图谱中,可观察到此连氧次甲基信号(δH 3.59)与4α-CH3(δC 23.7)/4β-CH3(δC 24.6)有远程相关,结合从1H-1H COSY图谱中观察到的H-1(δH 4.82)↔H-2β(2.15)↔H-3(3.59)质子自旋体系,提示化合物1是化合物2的3-羟基衍生物。该化合物的相对构型通过NOESY图谱确定,H-5β(δH 1.93)与4β-CH3(δH 1.04)、12b-CH3(δH 1.19)以及6a-CH3(δH 1.47)有NOE相关,提示4β-CH3、12b-CH3和6a-CH3为β构型;H-3(δH 3.59)与4β-CH3(δH 1.04)有NOE相关,提示H-3亦为β构型(图2)。
图2
图2
化合物1的关键1H-1H COSY、HMBC和NOE相关
Fig. 2
Key 1H-1H COSY, HMBC and NOE correlations of compound 1.
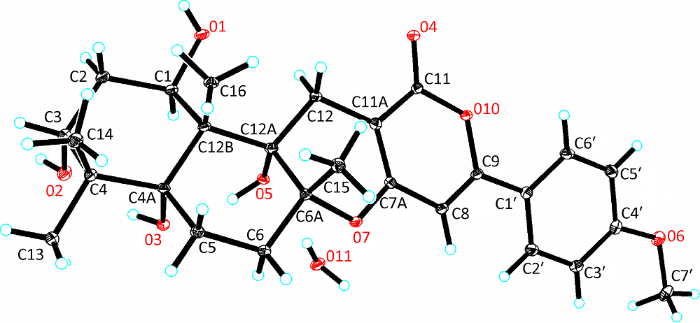
进一步通过单晶X衍射实验(Cu Kα)确定了化合物1的绝对构型为1R,3R,4aR, 6aR,12aS,12bS,Flack常数为0.00(7)。单晶数据如下:C27H36O9 (fw=504.56), orthorhombic, space group P212121, a=6.90730 (10) Å, b=17.2580 (2) Å, c=19.8227 (3) Å, V=2362.99 (6) Å3, Z=4, DC=1.418mg/mm3, F (000)=1080, 26717 reflections detected, 4764 independent reflections. R1=0.0353 and WR2=0.0903 for I>2σ(I), S=1.074, Flack parameter=0.00(7) (CCDC 1998884)(图3)。
图3
综合以上解析,确定化合物1的结构为一个新的杂萜类化合物,命名为asptercin A。
化合物2:白色针状晶体(甲醇);[α]25 D+23(c 0.1, CH3OH);HR-ESI-MS m/z: 471.2381 [M+H]+;分子式C27H34O7。1H NMR(400MHz,CD3OD)δH:7.80(2H, d, J=9.0Hz, H-2’/H-6’),7.02(2H, d, J=9.0Hz, H-3’/H-5’),6.57(1H, s, H-8),3.92(1H, dd, J=9.4, 7.8Hz, H-1),3.85(3H, s, 4’-OCH3),2.73(1H, d, J=16.9Hz, H-12β),2.38(1H, m, H-2β),2.37(1H, d, J=16.9Hz, H-12α),2.03(1H, m, H-5β),1.84-1.74(3H, overlapped, H-3β/H-6),1.72(1H, m, H-5α),1.48(3H, s, 6a-CH3),1.37(1H, m, H-3α),1.29(1H, m, H-2α),1.23(3H, s, 4β-CH3),1.03(3H, s, 4α-CH3),0.98(3H, s, 12b-CH3);13C NMR(100MHz,CD3OD)δC:167.3(C-11),166.1(C-7a),163.4(C-4’),159.9(C-9),128.2(C-2’/C-6’),125.0(C-1’),115.5(C-3’/C-5’),99.5(C-11a),98.2(C-8),83.3(C-4a),82.5(C-6a),78.2(C-12a),74.2(C-1),56.0(4’-OCH3),45.7(C-4),44.4(C-12b),30.6(C-2),27.8(C-6),26.9(C-3),26.5(C-12),26.3(C-5),25.0(6a-CH3),23.1(4α-CH3),21.2(4β-CH3),19.1(12b-CH3)。以上数据与Sunazuka et al.(2004)报道的数据一致,因此鉴定该化合物为(1R,4aR,6aR,12aR,12bS)-1,3,4,4a,5,6,6a, 12,12a,12b-decahydro-1,4a,12a-trihydroxy-4,4,6a,12b-tetramethyl-9-(4’-methoxyphenyl)-2H,11H-naphtho[2,1-b]pyrano[3,4-e]pyran-11-one(图1)。
化合物3:白色无定形粉末;[α]25 D+60(c 0.1, CH3OH);HR-ESI-MS m/z: 513.2488 [M+H]+;分子式C29H36O8。1H NMR(400MHz,CDCl3)δH:7.73(2H, d, J=8.9Hz, H-2’/H-6’),6.93(2H, d, J=9.0Hz, H-3’/H-5’),6.78(1H, d, J=2.8Hz, 12a-OH),6.34(1H, s, H-8),4.88(1H, t, J=2.9Hz, H-3),4.48(1H, s, 4a-OH),3.84(3H, s, 4’-OCH3),2.65(1H, dd, J=16.6, 2.9Hz, H-12α),2.46(1H, d, J=16.6Hz, H-12β),2.36(2H, overlapped, H-1α/H-6α),2.17(1H, m, H-2β),2.13[3H, s, 3-OAc (CH3)],1.85-1.79 (3H, overlapped, H-2α/H-5),1.75(1H, m, H-6β),1.44(3H, s, 6a-CH3),1.38(1H, ddd,J=14.1, 4.7, 2.5Hz, H-1β),1.18(3H, s, 12b-CH3),1.13(3H, s, 4β-CH3),1.02(3H, s, 4α-CH3);13C NMR(100MHz,CDCl3)δC:168.6[3-OAc (C=O)],165.2(C-11),163.3(C-7a),161.3(C-4’),158.1(C-9),127.0(C-2’/C-6’),124.1(C-1’),114.1(C-3’/C-5’),97.8(C-11a),96.9(C-8),81.5(C-4a/C-6a),79.0(C-3),76.5(C-12a),55.4(4’-OCH3),43.2(C-12b),41.8(C-4),29.0(C-6),25.8(C-12),25.0(C-5),24.7(6a-CH3),24.4(4β-CH3),22.8(4α-CH3),22.7(C-2),21.4(C-1),21.3[3-OAc (CH3)],21.2(12b-CH3)。以上数据与Otoguro et al.(2000)报道的arisugacin D一致,因此鉴定该化合物为arisugacin D(图1)。
化合物4:白色无定形粉末;[α]25 D+73(c 0.1, CH3OH);HR-ESI-MS m/z: 551.2255 [M+Na]+;分子式C29H36O9。1H NMR(400MHz,CDCl3)δH:7.74(2H, d, J=9.0Hz, H-2’/H-6’),6.94(2H, d, J=9.0Hz, H-3’/H-5’),6.34(1H, s, H-8),4.90(1H, d, J=1.7Hz, H-3),4.24(1H, dd, J=3.6, 2.4Hz, H-1),3.85(3H, s, 4’-OCH3),2.86(1H, dd, J=17.0, 2.5Hz, H-12α),2.67(1H, d, J=16.9Hz, H-12β),2.42(1H, m, H-2β),2.40(1H, m, H-6α),2.15(1H, m, H-2α),2.10[3H, s, 3-OAc (CH3)],1.86(2H, m, H-5),1.81(1H, m, H-6β),1.44(3H, s, 6a-CH3),1.13(3H, s, 4β-CH3),1.10(3H, s, 4α-CH3),1.06(3H, s, 12b-CH3);13C NMR(100MHz,CDCl3)δC:169.7[3-OAc (C=O)],164.9(C-11),163.3(C-7a),161.5(C-4’),158.6(C-9),127.1(C-2’/C-6’),123.9(C-1’),114.2(C-3’/C-5’),96.8(C-11a),96.6(C-8),81.3(C-4a),80.6(C-6a),79.3(C-12a),77.2(C-3),72.9(C-1),55.4(4’-OCH3),44.4(C-12b),42.2(C-4),29.3(C-2),28.9(C-6),25.9(C-12),25.4(C-5),24.7(6a-CH3),24.4(4β-CH3),22.9(4α-CH3),21.4[(3-OAc(CH3)],21.2(12b-CH3)。以上数据与Otoguro et al.(2000)报道的arisugacin H一致,因此鉴定该化合物为arisugacin H(图1)。
化合物5:白色无定形粉末;[α]25 D+92(c 0.1, CH3OH);HR-ESI-MS m/z: 533.1804 [M+Na]+;分子式C28H30O9。1H NMR(400MHz,CDCl3)δH:7.05(1H, d , J=1.6Hz, H-2’),6.92(1H, d, J=1.6Hz, H-6’),6.31(2H, overlapped, H-3/H-8),6.04(2H, s, OCH2O),5.83(1H, d, J=10.2Hz, H-2),3.95(3H, s, 5’-OCH3),3.37(1H, d, J=17.8Hz, H-12α),2.83(1H, d, J=17.8Hz, H-12β),2.46(1H, m, H-6α),1.92-1.87(2H, m, H-5),1.82(1H, m, H-6β),1.51(3H, s, 6a-CH3),1.45(3H, s, 12b-CH3),1.27(3H, s, 4α-CH3),1.18(3H, s, 4β-CH3);13C NMR(100MHz,CDCl3)δC:204.7(C-1),164.3(C-11),162.9(C-7a),158.4(C-9),153.5(C-3),149.3(C-3’),143.8(C-5’),137.6(C-4’),125.9(C-1’),123.3(C-2),105.7(C-2’),102.1(OCH2O),99.7(C-6’),97.1(C-8),97.0(C-11a),79.9(C-6a),79.0(C-4a),76.2(C-12a),56.7(5’-OCH3),56.2(C-12b),42.6(C-4),28.4(C-6),27.9(C-12),25.8(C-5),25.5(4α-CH3),23.9(4β-CH3),23.8(6a-CH3),21.8(12b-CH3)。以上数据与Lee et al.(1992)报道的territrem A一致,因此鉴定该化合物为territrem A(图1)。
化合物6:白色无定形粉末;[α]25 D+86(c 0.1, CH3OH);HR-ESI-MS m/z: 527.2284 [M+H]+;分子式C29H34O9。1H NMR(400MHz,CDCl3)δH:6.96(2H, s, H-2’/H-6’),6.34(1H, s, H-8),6.30(1H, d, J=10.2Hz, H-3),5.81(1H, d, J=10.2Hz, H-2),3.88(9H, overlapped, 3’-OCH3/4’-OCH3/5’-OCH3),3.41(1H, d, J=17.8Hz, H-12α),2.83(1H, d, J=17.8Hz, H-12β),2.45(1H, m, H-6α),2.16(1H, m, H-6β),1.82-1.74(2H, m, H-5),1.50(3H, s, 6a-CH3),1.44(3H, s, 12b-CH3),1.26(3H, s, 4α-CH3),1.17(3H, s, 4β-CH3);13 C NMR(100MHz,CDCl3)δC:204.5(C-1),164.4(C-11),162.8(C-7a),158.4(C-9),153.4(C-3),153.4(C-3’/C-5’),140.2(C-4’),126.7(C-1’),123.3(C-2),102.6(C-2’/C-6’),97.6(C-8),97.3(C-11a),80.0(C-6a),79.0(C-4a),76.1(C-12a),60.9(4’-OCH3),56.3(3’-OCH3/5’-OCH3),56.2(C-12b),42.6(C-4),28.4(C-6),27.8(C-12),25.7(C-5),25.4(4α-CH3),23.8(4β-CH3/6a-CH3),21.8(12b-CH3)。以上数据与Lee et al.(1992)报道的territrem B一致,因此鉴定该化合物为territrem B(图1)。
化合物7:白色无定形粉末;[α]25 D+84(c 0.1, CH3OH);HR-ESI-MS m/z: 535.1939 [M+Na]+;分子式C28H32O9。1H NMR(400MHz,CDCl3)δH:7.01(2H, s, H-2’/H-6’),6.32(1H, s, H-8),6.31(1H, d, J=10.2Hz, H-3),5.83(1H, m, H-2),3.93(6H, overlapped, 3’-OCH3/5’- OCH3),3.39(1H, d, J=17.7Hz, H-12α),2.84(1H, d, J=17.7Hz, H-12β),2.45(1H, m, H-6α),1.91-1.87(2H, m, H-5),1.81(1H, m, H-6β),1.51(3H, s, 6a-CH3),1.45(3H, s, 12b-CH3),1.27(3H, s,4α-CH3),1.18(3H, s, 4β-CH3);13C NMR(100MHz,CDCl3)δC:204.6(C-1),164.5(C-11),163.0(C-7a),158.8(C-9),153.5(C-3),147.1(C-3’/C-5’),137.2(C-4’),123.3(C-2),122.7(C-1’),102.4(C-2’/C-6’),96.7(C-8/C-11a),79.9(C-6a),79.0(C-4a),76.1(C-12a),56.5(3’-OCH3/5’- OCH3),56.2(C-12b),42.6(C-4),28.4(C-6),27.8(C-12),25.7(C-5),25.4(4α-CH3),23.8(4β-CH3/6a-CH3),21.8(12b-CH3)。以上数据与Lee et al.(1992)报道的territrem C一致,因此鉴定该化合物为territrem C(图1)。
2.2 乙酰胆碱酯酶抑制活性
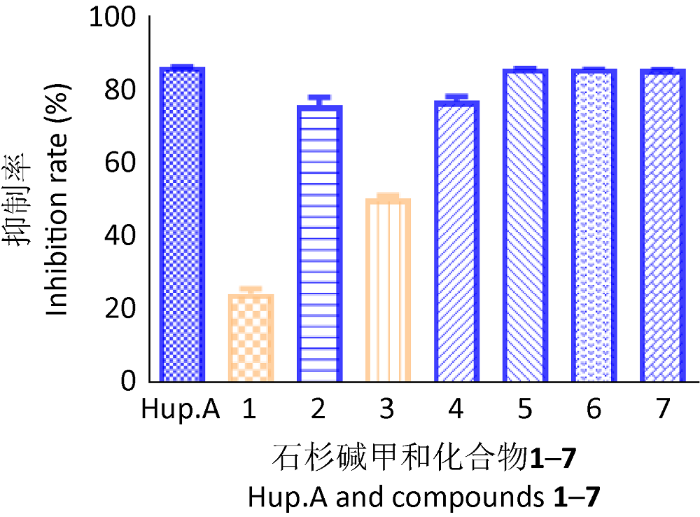
乙酰胆碱酯酶抑制活性结果显示,在0.1mmol/L的浓度下,化合物2、4、5、6和7的抑制率分别为76.1%、77.1%、86.1%、86.1%和86.0%;而化合物1和化合物3的抑制率分别为24.6%、50.6%;阳性对照石杉碱甲,在0.1mmol/L的浓度下,对乙酰胆碱酯酶的抑制率为86.5%(IC50为0.099μmol/L)(图4)。
图4
3 讨论
通过对分离自泰国马六甲木果楝Xylocarpus moluccensis中的一株土曲霉Aspergillus terreus xy03发酵物的次生代谢产物进行研究,分离鉴定出7个杂萜类化合物,其中1为新化合物,命名为asptercin A。化合物2、4、5、6和7具有良好的乙酰胆碱酯酶抑制活性,其抑制率分别为76.1%、77.1%、86.1%、86.1%和86.0%。本研究丰富了红树内生真菌的化学成分内涵。
致谢:
感谢南方医科大学吴军教授、中国医学科学院北京协和医学院药物研究所戴均贵研究员提供实验菌株。
参考文献
Spiro fused diterpene-indole alkaloids from a creek-bottom-derived Aspergillus terreus
DOI:10.1021/ol401891z
URL
[本文引用: 1]

Four metabolites, teraspiridoles A-D (2-5), formed from the merger of a diterpene and modified indole scaffold were obtained from an Aspergillus terreus isolate. The structures and absolute configurations of these natural products were established using NMR, mass spectrometry, Marfey's method, VCD, and ECD data. Teraspiridole B (3) exhibited weak inhibition of planaria regeneration/survival.
Mangrove derived fungal endophytes - a chemical and biological perception
DOI:10.1007/s13225-013-0243-8
URL
[本文引用: 1]

The potential of mangrove-derived endophytic fungi as a promising source of diverse and structurally unprecedented bioactive natural products is unquestionable and continues to attract considerable attention. This review highlights new bioactive mangrove fungal metabolites and known compounds with hitherto unreported biological activities described during the last 10 years. The compounds are categorized according to their reported biological activities, including cytotoxic, anti-infective, in addition to a wide range of miscellaneous activities such as protein kinase, alpha-glucosidase, acetylcholinesterase and tyrosinase inhibitory activities, as well as antiangiogenic and neovascularisation effects, radical scavenging, DNA-binding affinity, and calcium and potassium channel blocking activity.
Terrenolide S, a new antileishmanial butenolide from the endophytic fungus Aspergillus terreus
DOI:10.1080/14786419.2015.1072711
URL
PMID:26299734
[本文引用: 1]

Terrenolide S, a new butenolide derivative (6), together with six known compounds: (22E,24R)-stigmasta-5,7,22-trien-3-beta-ol (1), stigmast-4-ene-3-one (2), stigmasta-4,6,8(14),22-tetraen-3-one (3), terretonin A (4), terretonin (5) and butyrolactone VI (7) have been isolated from the endophytic fungus Aspergillus terreus isolated from the roots of Carthamus lanatus (Asteraceae). Their structures were established by extensive spectroscopic analyses (1D, 2D NMR and HRESIMS), as well as optical rotation measurement and comparison with literature data. Compound 1 displayed a potent activity towards methicillin-resistant Staphylococcus aureus (MRSA) and Cryptococcus neoformans with IC50 values of 2.29 and 10.68 microM, respectively. Moreover, 1, 2 and 6 exhibited antileishmanial activity towards Leishmania donovani with IC50 values of 11.24, 15.32 and 27.27 microM, respectively and IC90 values of 14.68, 40.56 and 167.03 microM, respectively.
A new and rapid colorimetric determination of acetylcholinesterase activity
DOI:10.1016/0006-2952(61)90145-9 URL [本文引用: 1]
Butenolide derivatives from the plant endophytic fungus Aspergillus terreus
DOI:10.1016/j.fitote.2016.06.014
URL
PMID:27370101
[本文引用: 1]

Three new butenolides containing 5-hydroxyfuran-2(5H)-one core, asperteretal A (1), asperteretal B (2), and asperteretal C (3), together with seven known butenolides (4-10), were obtained from an endophytic fungus Aspergillus terreus PR-P-2 isolated from the plant Camellia sinensis var. assamica. The structures of compounds 1-3 were elucidated on the basis of detailed spectroscopic analysis including UV, IR, HRESIMS, 1D and 2D NMR, and ECD spectra. Compounds 1, 3, 5 and 6-8 showed potent inhibitory effects on NO production in RAW 264.7 lipopolysaccharide-induced macrophages, and compounds 5 and 8 also exhibited moderate cytotoxicity against HL-60 cell line.
Xanthone-derived polyketides from the thai mangrove endophytic fungus Phomopsis sp. xy21
NMR assignments of territrems A, B, and C and the structure of MB2, the major metabolite of territrem B by rat liver microsomal fraction
Diversity and in vitro antitumor activity of endophytic fungi from mangrove plants Xylocarpus
A total of 24 biologically pure entophytic fungal strains were isolated from stems, leaves, and seed coats of Xylocarpus plants by repeated purification, and identified with Internal Transcribed Spacer (ITS) rDNA molecular method, which belonging to 14 genera, 11 families, 9 orders and 3 classes. There were differences in genus and species levels among three plant materials from different habitats and species, and it was found that the strains of Phomopsis and Colletotrichum existed in all three plant materials. In vitro assay of antitumor activity by MTT method revealed that the EtOAc extracts of 15 strains exhibited potent antitumor activity. These results suggest that it is of value for further investigation on the above fungal strains.
New meroterpenoids from Aspergillus terreus with inhibition of cyclooxygenase-2 expression
DOI:10.1021/acs.orglett.5b00739
URL
PMID:25915447
[本文引用: 1]

Two novel meroterpenoids, yaminterritrems A (1) and B (2), were isolated from Aspergillus terreus collected from hot spring zones in Yang-Ming Mountain, Taiwan, and cultured at 40 degrees C. The structures of 1 and 2 were elucidated by NMR, MS spectral and X-ray crystallographic analyses. The biosynthetic route for 1 and 2 involving the conversion of the sesquiterpene with phenyl-alpha-pyrone is proposed. Besides, 2 exhibited a dose-dependent inhibitory effect on COX-2 expression in LPS-stimulated RAW264.7 macrophages.
Aspterpenacids A and B, two sesterterpenoids from a mangrove endophytic fungus Aspergillus terreus H010
Cytochalasins from mangrove endophytic fungi Phomopsis spp. xy21 and xy22
DOI:10.1016/j.phytol.2016.07.027 URL [本文引用: 1]
Uncovering the unusual D-ring construction in terretonin biosynthesis by collaboration of a multifunctional cytochrome P450 and a unique isomerase
DOI:10.1021/jacs.5b00570
URL
PMID:25671343
[本文引用: 1]

Terretonin (1) is a fungal meroterpenoid isolated from Aspergillus terreus, and possesses a highly oxygenated and unique tetracyclic structure. Although the biosynthetic gene cluster for 1 has been identified and the biosynthesis has recently been studied by heterologous reconstitution and targeted-gene deletion experiments, the last few steps of the terretonin pathway after terrenoid (6) have yet to be elucidated. Notably, the mechanism for the D-ring expansion to afford the terretonin scaffold has been a long-standing mystery to solve. Here we report the characterization of three enzymes that convert 6 into 1, as well as the complete biosynthetic pathway of 1. In the proposed terretonin pathway, the cytochrome P450 Trt6 catalyzes three successive oxidations to transform 6 into an unstable intermediate, which then undergoes the D-ring expansion and unusual rearrangement of the methoxy group to afford the core skeleton of 1. This unprecedented rearrangement is catalyzed by a novel isomerase Trt14. Finally, the nonheme iron-dependent dioxygenase Trt7 accomplishes the last two oxidation reactions steps to complete the biosynthesis.
Territrem and butyrolactone derivatives from a marine-derived fungus Aspergillus terreus
DOI:10.3390/md12126113
URL
PMID:25522319
[本文引用: 1]

Seventeen lactones including eight territrem derivatives (1-8) and nine butyrolactone derivatives (9-17) were isolated from a marine-derived fungus Aspergillus terreus SCSGAF0162 under solid-state fermentation of rice. Compounds 1-3 and 9-10 were new, and their structures were elucidated by spectroscopic analysis. The acetylcholinesterase inhibitory activity and antiviral activity of compounds 1-17 were evaluated. Among them, compounds 1 and 2 showed strong inhibitory activity against acetylcholinesterase with IC50 values of 4.2 +/- 0.6, 4.5 +/- 0.6 nM, respectively. This is the first time it has been reported that 3, 6, 10, 12 had evident antiviral activity towards HSV-1 with IC50 values of 16.4 +/- 0.6, 6.34 +/- 0.4, 21.8 +/- 0.8 and 28.9 +/- 0.8 mug.mL-1, respectively. Antifouling bioassay tests showed that compounds 1, 11, 12, 15 had potent antifouling activity with EC50 values of 12.9 +/- 0.5, 22.1 +/- 0.8, 7.4 +/- 0.6, 16.1 +/- 0.6 mug.mL-1 toward barnacle Balanus amphitrite larvae, respectively.
Arisugacins C and D, novel acetylcholinesterase inhibitors and their related novel metabolites produced by Penicillium sp. FO-4259-11
DOI:10.7164/antibiotics.53.50 URL [本文引用: 2]
Studies on the chemical constituents and bioactivities of Aspergillus terreus
Asperterpenes A and B, two unprecedented meroterpenoids from Aspergillus terreus with BACE1 inhibitory activities
DOI:10.1039/c6sc02464e
URL
PMID:28042460
[本文引用: 1]

Asperterpenes A (1) and B (2), two 3,5-dimethylorsellinic acid-based meroterpenoids that contain a unique beta-oriented Me-21 with an unprecedented 1,2,5-trimethyl-4,9-dioxobicyclo[3.3.1]non-2-ene-3-carboxylic acid moiety, were obtained from Aspergillus terreus in very limited amounts of 3.6 mg and 1.8 mg, respectively. The absolute structure of 1 was determined using X-ray diffraction. Because of the low yield of 1, a comprehensive characterization of the BACE1 inhibitory activities of 1 was completed via molecular biological, cell and animal studies guided by in silico target confirmation (ISTC). ISTC assays suggested that compounds 1 and 2 might be BACE1 inhibitors. In cell-based tests, asperterpenes A and B, as natural products, exhibited promising inhibitory activities against BACE1, with IC50 values of 78 and 59 nM, respectively. LY2811376 (the positive control), one of the most potent clinical BACE1 inhibitors, has shown an IC50 value of 260 nM. In vivo, compound 1 exhibited activity similar to that of LY2811376 against Alzheimer's disease (AD) in 3xTg AD mice. Taken together, these findings demonstrate that asperterpene A, which contains a novel carbon skeleton, is the first terpenoid to exhibit effective BACE1 inhibitory activity. Moreover, 1 represents a potential lead compound and a versatile scaffold for the development of drugs for the treatment of AD.
Absolute stereochemistries and total synthesis of (+)-arisugacins A and B, potent, orally bioactive and selective inhibitors of acetylcholinesterase
DOI:10.1016/j.tet.2004.06.059
URL
[本文引用: 1]

Abstract
In the current studies, we used the Kakisawa–Kashman modification of the Mosher NMR method to determine the complete absolute stereochemistry of arisugacins. We also report the convergent total synthesis of (+)-arisugacins A and B by a sequence including (i) ruthenium complex-catalyzed asymmetric reduction of the cyclohexenone derivative; (ii) stereoselective construction of the arisugacin skeleton by a Knoevenagel-type reaction of an α,β-unsaturated aldehyde derivative with production of a 4-hydroxy-2-pyrone derivative as a key reaction; and (iii) stereoselective dihydroxylation to give the diol derivative, followed by deoxygenation. Accordingly, we defined the absolute structures of arisugacins A and B as 4a-(R),6a-(R),12a-(R), and 12b-(S). Finally, we characterized the bioactivities of the synthetic intermediates to understand the structure–activity relationships of the arisugacins.
Graphic
Three xanthone dimers from the thai mangrove endophytic fungus Phomopsis sp. xy21
DOI:10.1080/10286020.2017.1381089 URL [本文引用: 1]
Resource diversity of Chinese macrofungi: edible, medicinal and poisonous species
Alkaloids and polyketides from the soil fungus Aspergillus terreus and their antibacterial activities
DOI:10.1007/s10600-017-2243-5 URL [本文引用: 1]
Isoterreulactone A, a novel meroterpenoid with anti-acetylcholinesterase activity produced by Aspergillus terreus
DOI:10.1016/j.bmcl.2004.10.067
URL
PMID:15603953
[本文引用: 1]

A new seven-membered lactone type meroterpenoid, isoterreulactone A, was isolated from the solid state fermentation of Aspergillus terreus and its structure was established by various spectral analysis. Isoterreulactone A inhibited acetylcholinesterase with an IC(50) value of 2.5 microM while did not inhibit butyrylcholinesterase even at 500 microM.
红树林木果楝属植物可培养内生真菌的多样性及其代谢产物抗肿瘤活性