Using environmental niche models to test the ‘everything is everywhere’ hypothesis for Badhamia
1
2014
... 黏菌形态种下可能存在多个隐存种,这些隐存种之间彼此存在生殖隔离,但难以从形态上区分(Feng & Schnittler 2015;Feng et al. 2016),进而干扰所研究物种的地理分布模式.目前,在许多黏菌的生物地理分布研究中增加了分子标记手段,通过基因型的差异更准确地反映物种分布,但结论也不尽相同.Winsett & Stephenson(2011)以mt SSU rRNA基因分析了畸形钙皮菌Didymium difforme的地理分布规律,13种mt SSU rRNA基因型为随机分布.Hoppe(2013)研究显示煤绒菌黄色变种Fuligo septica var. flava可根据mt SSU rRNA基因分为3个分支,分支间的遗传距离与地理距离不相关.Aguilar et al.(2014)采用SSU rRNA基因系统发育分析和环境生态位模拟,分析了美洲的暗孢钙丝菌Badhamia melanospora种群遗传分化,结果显示,南北美洲的暗孢钙丝菌B. melanospora按照大陆的分离完全隔断为南北两个基因型的种群,大部分种群局限分布在一个较为固定的地理范围内.Dagamac et al.(2017c)通过SSU rRNA基因序列分析发现蛇形半网菌Hemitrichia serpula包含3个主要的系统发育群,不同种群的地理分布、形态特征和EF-1α基因序列也显著不同.Janik et al.(2020)研究了雪生钙皮菌Didymium nivicola的SSU rRNA和EF-1α基因多态性,发现在安第斯山脉的种内遗传多样性最高,包含17种SSU核糖体型和12种EF-1α基因型,而北半球种群的遗传背景则比较一致,因此认为安第斯山脉可能是雪生钙皮菌D. nivicola的遗传多样性分化中心. ...
Introductory mycology. 4th ed
2
1996
... 自Panckow在1654年首次记录并描述粉瘤菌Lycogala epidendrum开始,尤其是Rostafinski在1873年建立第一个黏菌现代分类系统以来(Alexopoulos et al. 1996),黏菌工作者陆续在世界各地开展了大量的标本采集和经典分类的研究工作,出版了众多专著.但在很长一段时期内,研究区域主要集中于北半球的温带地区,其中以北美、欧洲和亚洲的温带森林生态系统最为突出(Stephenson et al. 2008),不仅有众多的论文,而且有许多地区志,包括北美的《The North American slime-moulds》和《The Mycetozoa of North America》,欧洲的《The Myxomycetes of Great Britain》《A monograph of the Mycetozoa— Being a descriptive catalogue of the species in the herbarium of the British Museum》《Mycological flora of European and Asian Russia. The slime molds》《A guide to temperate Myxomycetes》《Die Myxomyceten Deutschlands und des angrenzenden Alpenraumes unter besonderer Berücksichtigung Österreichs》《The Myxomycetes of Britain and Ireland. An identification handbook》和《Les Myxomycètes》,亚洲的《The Myxomycetes of Japan》《The Myxomycetes of India》《Taxonomy of the Indian Myxomycetes》《The myxomycete biota of Japan》和《中国真菌志·黏菌卷》等等.从已有调查结果来看,温带落叶阔叶林可能是地球上黏菌物种多样性最高的生态系统,如美国大雾山国家公园报道黏菌168种(Stephenson et al. 2001);俄罗斯锡霍特-阿林保护区报道黏菌158种(Novozhilov et al. 2017b);中国小兴安岭地区报道黏菌152种(赵凤云等 2019). ...
... 原生动物的生物地理分布模式,存在两种假说:一为原生动物在全球范围内呈随机分布,其分布主要由所处生境中的环境因子决定,即“everything is everywhere, but the environment selects”(Finlay 2002);二为与之相对应的地带性分布(moderate endemicity),指物种的分布受扩散限制的影响(Foissner 2006).黏菌归属于原生动物界已经获得普遍接受(Alexopoulos et al. 1996),其孢子能够借助气流进行长距离散播(Kamono et al. 2009a),具有较强的扩散能力.基于标本采集的物种调查和形态分类学研究结果一方面为黏菌的随机分布提供了证据支持,如许多黏菌物种呈现出跨大陆的分布格局(Stephenson et al. 2008).黏菌物种调查研究同时显示,偏远海岛的物种组成与大陆类似而无特有种存在,包括美国夏威夷群岛(Eliasson 1991)和澳大利亚麦夸里岛(Stephenson et al. 2007)等.另一方面,一些研究结果表明黏菌也可能受扩散限制的影响,而呈现地带性分布,如裂瓣菌Barbeyella minutissima集中分布于温带森林,而圆孢鹅绒菌Ceratiomyxa sphaerosperma仅知分布于美洲的热带地区(Stephenson & Rojas 2017).Dagamac et al.(2017b)研究了新热带和古热带地区两种森林生态系统的黏菌群落组成,发现一些物种的分布局限于特定的地理区域,地理距离而非生境差异对黏菌群落组成影响更大. ...
Different degrees of niche differentiation for bacteria, fungi, and myxomycetes within an elevational transect in the German Alps
2
2019
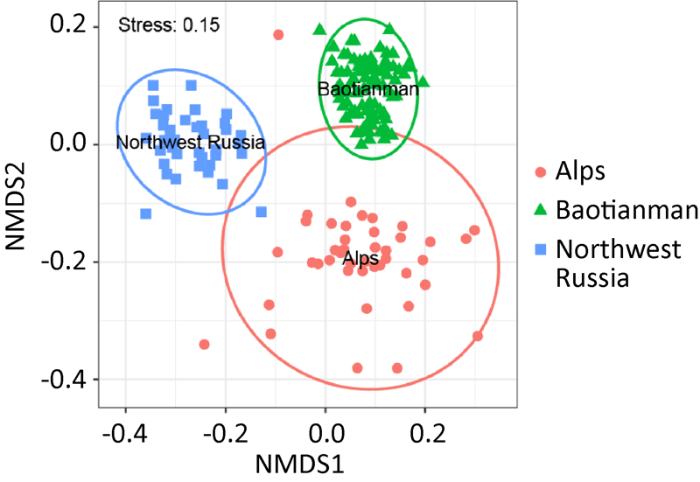
... 高通量测序技术的应用极大地增加了扩增子测序的测序深度,目前从草原(Fiore- Donno et al. 2016)、融雪带(Borg Dahl et al. 2018b)和森林(Borg Dahl et al. 2019;Gao et al. 2019;Shchepin et al. 2019)土壤中所报道的暗孢黏菌OTUs数量在200-300之间.在群落组成方面,已有18属暗孢黏菌报道分布于土壤中,其中亮皮菌属Lamproderma、发网菌属Stemonitis、裂皮菌属Meriderma和双皮菌属Diderma为优势属,一些典型的林中凋落物栖生黏菌如高杯菌Craterium minutum、光果菌Leocarpus fragilis等也被发现分布于土壤中(Gao et al. 2019;Shchepin et al. 2019).土壤中黏菌的分布格局也得到进一步认识,例如,森林火灾后不同年份土壤的黏菌组成显著不同,表明黏菌群落存在显著的演替规律;不同植被土壤的黏菌群落存在显著差异,并主要由土壤pH驱动(Borg Dahl et al. 2019;Gao et al. 2019).我们整合了目前已发表的土壤暗孢黏菌高通量测序数据(PRJNA418896;PRJNA491744;PRJNA565474),通过非度量多维尺度(non-metric multidimensional scaling,NMDS)分析发现,德国阿尔卑斯山地森林、俄罗斯西北部泰加林和中国河南宝天曼保护区温带森林的土壤黏菌群落组成显著不同(图2),这也为黏菌的大尺度地带性分布格局提供了佐证. ...
... ).土壤中黏菌的分布格局也得到进一步认识,例如,森林火灾后不同年份土壤的黏菌组成显著不同,表明黏菌群落存在显著的演替规律;不同植被土壤的黏菌群落存在显著差异,并主要由土壤pH驱动(Borg Dahl et al. 2019;Gao et al. 2019).我们整合了目前已发表的土壤暗孢黏菌高通量测序数据(PRJNA418896;PRJNA491744;PRJNA565474),通过非度量多维尺度(non-metric multidimensional scaling,NMDS)分析发现,德国阿尔卑斯山地森林、俄罗斯西北部泰加林和中国河南宝天曼保护区温带森林的土壤黏菌群落组成显著不同(图2),这也为黏菌的大尺度地带性分布格局提供了佐证. ...
Genetic barcoding of dark‐spored myxomycetes (Amoebozoa)—identification, evaluation and application of a sequence similarity threshold for species differentiation in NGS studies
1
2018a
... 早期的黏菌生物学围绕黏菌生活史开展了大量研究,其中一些研究揭示了孢子萌发、黏变形体生长、原生质团行为等与所处的生态环境,包括温度、湿度、pH等一系列环境因素之间的关系(Madelin 1984),可以将这些研究归纳到个体生态学的范畴.Maimoni- Rodella & Gottsberger(1980)定量分析了巴西热带森林中的黏菌群落与基质类型、温度和降水量的关系,开启了黏菌的群落生态学研究.目前,绝大多数黏菌生态学研究首先通过子实体调查和分类鉴定,获得一定区域或生境内的黏菌物种组成与丰度信息;再应用数量生态学的分析手段,揭示黏菌群落的物种分布格局和影响因素等(Stephenson & Rojas 2017).这种基于子实体调查的方法不可避免地忽略了环境中一些无法发育为子实体的黏菌营养体(李玉等 2021),因此,许多研究者尝试将DNA分子标记和指纹图谱技术用于解析环境中的黏菌群落,如PCR-DGGE(Kamono & Fukui 2006;Kamono et al. 2009b)、PCR-T-RFLP(Hoppe & Schnittler 2015)等,标志着黏菌生态学研究已进入分子时代.当前,黏菌DNA条形码与高通量测序结合产生的扩增子高通量测序为黏菌生态学研究提供了新方法,通过环境基因组DNA提取、条形码基因PCR扩增、产物高通量测序和序列分类注释,已实现了不依赖子实体调查的群落定性、定量分析(Borg Dahl et al. 2018a). ...
A four year survey reveals a coherent pattern between occurrence of fruit bodies and soil amoebae populations for nivicolous myxomycetes
1
2018b
... 高通量测序技术的应用极大地增加了扩增子测序的测序深度,目前从草原(Fiore- Donno et al. 2016)、融雪带(Borg Dahl et al. 2018b)和森林(Borg Dahl et al. 2019;Gao et al. 2019;Shchepin et al. 2019)土壤中所报道的暗孢黏菌OTUs数量在200-300之间.在群落组成方面,已有18属暗孢黏菌报道分布于土壤中,其中亮皮菌属Lamproderma、发网菌属Stemonitis、裂皮菌属Meriderma和双皮菌属Diderma为优势属,一些典型的林中凋落物栖生黏菌如高杯菌Craterium minutum、光果菌Leocarpus fragilis等也被发现分布于土壤中(Gao et al. 2019;Shchepin et al. 2019).土壤中黏菌的分布格局也得到进一步认识,例如,森林火灾后不同年份土壤的黏菌组成显著不同,表明黏菌群落存在显著的演替规律;不同植被土壤的黏菌群落存在显著差异,并主要由土壤pH驱动(Borg Dahl et al. 2019;Gao et al. 2019).我们整合了目前已发表的土壤暗孢黏菌高通量测序数据(PRJNA418896;PRJNA491744;PRJNA565474),通过非度量多维尺度(non-metric multidimensional scaling,NMDS)分析发现,德国阿尔卑斯山地森林、俄罗斯西北部泰加林和中国河南宝天曼保护区温带森林的土壤黏菌群落组成显著不同(图2),这也为黏菌的大尺度地带性分布格局提供了佐证. ...
First insight into dead wood protistan diversity: a molecular sampling of bright-spored Myxomycetes (Amoebozoa, slime-moulds) in decaying beech logs
1
2015
... Feest & Madelin(1985)最早通过计数培养后获得的黏菌原生质团定量分析了土壤中分布的黏变形体,研究结果表明,黏菌的黏变形体普遍栖生于森林、草原和农田等不同类型的土壤中,丰度约为8 000个/cm3,其季节动态与细菌丰富相关(Feest & Madelin 1988).在采用分子生物学方法开展的研究中,Kamono & Fukui(2006)将PCR-DGGE技术应用于分析土壤绒泡菌目Physarales的营养体群落,发现不同深度土层的黏菌种类存在差异,但未检测到明显的季节动态变化(Kamono et al. 2009b).在后续研究中,Kamono et al.(2013)以SSU rRNA基因扩增子克隆测序的方法分析了雪线土壤中的暗孢黏菌多样性,序列注释结果表明,亮皮菌属Lamproderma、双皮菌属Diderma和钙皮菌属Didymium为优势类群,来自法国、苏格兰和日本的土壤样品中,黏菌物种组成显著不同.在腐木中,Clissmann et al.(2015)获得29种亮孢黏菌的可操作分类单元(operational taxa units,OTUs),序列注释结果表明,团网菌属Arcyria、团毛菌属Trichia和粉瘤菌属Lycogala是优势属,腐木的pH是决定黏菌群落分布的主要环境因子. ...
A comparative species listing of myxomycetes from tropical (Philippines) and temperate (United States) forests
1
2014
... 黏菌的纬度分布格局研究表明,黏菌的物种丰富度与纬度呈正相关关系,林型、土壤理化性质和基质pH是这一分布格局的主要驱动因子(Rojas et al. 2011).同时,在温带与热带森林黏菌多样性的比较研究中,温带森林均高于热带森林,热带地区过高的降水量和林下快速降解的凋落物不利于黏菌子实体的形成,同时,封闭的森林冠层使气流减少也可能会阻碍黏菌孢子的扩散(Stephenson et al. 1993;Cruz et al. 2014). ...
List of species collected and interactive database of myxomycetes (plasmodial slime molds) for Mt. Arayat National Park, Pampanga, Philippines
1
2011
... 热带、亚热带黏菌的早期研究仅限于零星报道.20世纪70年代,Farr(1976)系统整理了前人文献资料并记述了新热带区280种黏菌.在此基础上,Lado & Wrigley de Basanta(2008)重新梳理了分布于新热带地区的黏菌物种,共计431种.近年,研究人员陆续在我国热带和亚热带森林(闫淑珍等2012;Liu et al. 2013;魏滨等 2016;Gao et al. 2018;Li et al. 2021)、东南亚热带雨林开展了系统调查(Tran et al. 2006;2008;Ko Ko et al. 2010,2012,2013;Dagamac et al. 2011,2014,2017a;Novozhilov et al. 2017a,2020),极大地丰富了黏菌物种的地理分布和区系特征. ...
Rapid assessment of myxomycete diversity in the Bicol Peninsula, Philippines
1
2017a
... 热带、亚热带黏菌的早期研究仅限于零星报道.20世纪70年代,Farr(1976)系统整理了前人文献资料并记述了新热带区280种黏菌.在此基础上,Lado & Wrigley de Basanta(2008)重新梳理了分布于新热带地区的黏菌物种,共计431种.近年,研究人员陆续在我国热带和亚热带森林(闫淑珍等2012;Liu et al. 2013;魏滨等 2016;Gao et al. 2018;Li et al. 2021)、东南亚热带雨林开展了系统调查(Tran et al. 2006;2008;Ko Ko et al. 2010,2012,2013;Dagamac et al. 2011,2014,2017a;Novozhilov et al. 2017a,2020),极大地丰富了黏菌物种的地理分布和区系特征. ...
Biogeographical assessment of myxomycete assemblages from Neotropical and Asian Palaeotropical forests
1
2017b
... 原生动物的生物地理分布模式,存在两种假说:一为原生动物在全球范围内呈随机分布,其分布主要由所处生境中的环境因子决定,即“everything is everywhere, but the environment selects”(Finlay 2002);二为与之相对应的地带性分布(moderate endemicity),指物种的分布受扩散限制的影响(Foissner 2006).黏菌归属于原生动物界已经获得普遍接受(Alexopoulos et al. 1996),其孢子能够借助气流进行长距离散播(Kamono et al. 2009a),具有较强的扩散能力.基于标本采集的物种调查和形态分类学研究结果一方面为黏菌的随机分布提供了证据支持,如许多黏菌物种呈现出跨大陆的分布格局(Stephenson et al. 2008).黏菌物种调查研究同时显示,偏远海岛的物种组成与大陆类似而无特有种存在,包括美国夏威夷群岛(Eliasson 1991)和澳大利亚麦夸里岛(Stephenson et al. 2007)等.另一方面,一些研究结果表明黏菌也可能受扩散限制的影响,而呈现地带性分布,如裂瓣菌Barbeyella minutissima集中分布于温带森林,而圆孢鹅绒菌Ceratiomyxa sphaerosperma仅知分布于美洲的热带地区(Stephenson & Rojas 2017).Dagamac et al.(2017b)研究了新热带和古热带地区两种森林生态系统的黏菌群落组成,发现一些物种的分布局限于特定的地理区域,地理距离而非生境差异对黏菌群落组成影响更大. ...
Speciation in progress? A phylogeographic study among populations of Hemitrichia serpula (Myxomycetes)
1
2017c
... 黏菌形态种下可能存在多个隐存种,这些隐存种之间彼此存在生殖隔离,但难以从形态上区分(Feng & Schnittler 2015;Feng et al. 2016),进而干扰所研究物种的地理分布模式.目前,在许多黏菌的生物地理分布研究中增加了分子标记手段,通过基因型的差异更准确地反映物种分布,但结论也不尽相同.Winsett & Stephenson(2011)以mt SSU rRNA基因分析了畸形钙皮菌Didymium difforme的地理分布规律,13种mt SSU rRNA基因型为随机分布.Hoppe(2013)研究显示煤绒菌黄色变种Fuligo septica var. flava可根据mt SSU rRNA基因分为3个分支,分支间的遗传距离与地理距离不相关.Aguilar et al.(2014)采用SSU rRNA基因系统发育分析和环境生态位模拟,分析了美洲的暗孢钙丝菌Badhamia melanospora种群遗传分化,结果显示,南北美洲的暗孢钙丝菌B. melanospora按照大陆的分离完全隔断为南北两个基因型的种群,大部分种群局限分布在一个较为固定的地理范围内.Dagamac et al.(2017c)通过SSU rRNA基因序列分析发现蛇形半网菌Hemitrichia serpula包含3个主要的系统发育群,不同种群的地理分布、形态特征和EF-1α基因序列也显著不同.Janik et al.(2020)研究了雪生钙皮菌Didymium nivicola的SSU rRNA和EF-1α基因多态性,发现在安第斯山脉的种内遗传多样性最高,包含17种SSU核糖体型和12种EF-1α基因型,而北半球种群的遗传背景则比较一致,因此认为安第斯山脉可能是雪生钙皮菌D. nivicola的遗传多样性分化中心. ...
The occurrence of litter myxomycetes at different elevations in Mt. Arayat, National Park, Pampanga, Philippines
1
2014
... 热带、亚热带黏菌的早期研究仅限于零星报道.20世纪70年代,Farr(1976)系统整理了前人文献资料并记述了新热带区280种黏菌.在此基础上,Lado & Wrigley de Basanta(2008)重新梳理了分布于新热带地区的黏菌物种,共计431种.近年,研究人员陆续在我国热带和亚热带森林(闫淑珍等2012;Liu et al. 2013;魏滨等 2016;Gao et al. 2018;Li et al. 2021)、东南亚热带雨林开展了系统调查(Tran et al. 2006;2008;Ko Ko et al. 2010,2012,2013;Dagamac et al. 2011,2014,2017a;Novozhilov et al. 2017a,2020),极大地丰富了黏菌物种的地理分布和区系特征. ...
The myxomycete biota of the Hawaiian Islands
1
1991
... 原生动物的生物地理分布模式,存在两种假说:一为原生动物在全球范围内呈随机分布,其分布主要由所处生境中的环境因子决定,即“everything is everywhere, but the environment selects”(Finlay 2002);二为与之相对应的地带性分布(moderate endemicity),指物种的分布受扩散限制的影响(Foissner 2006).黏菌归属于原生动物界已经获得普遍接受(Alexopoulos et al. 1996),其孢子能够借助气流进行长距离散播(Kamono et al. 2009a),具有较强的扩散能力.基于标本采集的物种调查和形态分类学研究结果一方面为黏菌的随机分布提供了证据支持,如许多黏菌物种呈现出跨大陆的分布格局(Stephenson et al. 2008).黏菌物种调查研究同时显示,偏远海岛的物种组成与大陆类似而无特有种存在,包括美国夏威夷群岛(Eliasson 1991)和澳大利亚麦夸里岛(Stephenson et al. 2007)等.另一方面,一些研究结果表明黏菌也可能受扩散限制的影响,而呈现地带性分布,如裂瓣菌Barbeyella minutissima集中分布于温带森林,而圆孢鹅绒菌Ceratiomyxa sphaerosperma仅知分布于美洲的热带地区(Stephenson & Rojas 2017).Dagamac et al.(2017b)研究了新热带和古热带地区两种森林生态系统的黏菌群落组成,发现一些物种的分布局限于特定的地理区域,地理距离而非生境差异对黏菌群落组成影响更大. ...
Myxomycetes associated with dryland ecosystems of the Tehuacán-Cuicatlán Valley Biosphere Reserve, Mexico
1
2009
... 2003年,随着“全球黏菌生物多样性”(PBI: Global Biodiversity of Eumycetozoans)项目的实施,黏菌分类和多样性研究也拓展至中亚和南美沙漠.出乎意料的是,相关研究借助湿室培养技术,揭示出这一生态系统中具有极高的黏菌物种多样性(Novozhilov et al. 2006;Novozhilov & Schnittler 2008;Estrada-Torres et al. 2009;Lado et al. 2011;2013;2014;Schnittler et al. 2013;Wrigley de Basanta et al. 2013).另外,一些特殊生境中分布的黏菌也引起广泛关注,如发生于春季融雪带的雪线黏菌陆续报道于西班牙格雷多山(Lado et al. 2005)、比利牛斯山(Lado & Ronikier 2008,2009)、波兰喀尔巴阡山(Ronikier et al. 2008)、澳大利亚科西阿斯科山(Stephenson & Shadwick 2009)和俄罗斯高加索山(Novozhilov et al. 2012)等山脉.在极地生态系统开展的黏菌调查研究表明,阿拉斯加、冰岛、格陵兰岛和俄罗斯等北极地区广泛分布着北温带的稀有种(Stephenson et al. 2000);类似地,亚南极地区麦夸里岛也发现了一些罕见种(Wrigley de Basanta et al. 2010),表明极地生态系统拥有较高的黏菌物种特有性. ...
Flora neotropica monograph No. 16 (Myxomycetes)
1
1976
... 热带、亚热带黏菌的早期研究仅限于零星报道.20世纪70年代,Farr(1976)系统整理了前人文献资料并记述了新热带区280种黏菌.在此基础上,Lado & Wrigley de Basanta(2008)重新梳理了分布于新热带地区的黏菌物种,共计431种.近年,研究人员陆续在我国热带和亚热带森林(闫淑珍等2012;Liu et al. 2013;魏滨等 2016;Gao et al. 2018;Li et al. 2021)、东南亚热带雨林开展了系统调查(Tran et al. 2006;2008;Ko Ko et al. 2010,2012,2013;Dagamac et al. 2011,2014,2017a;Novozhilov et al. 2017a,2020),极大地丰富了黏菌物种的地理分布和区系特征. ...
A method for the enumeration of myxomycetes in soils and its application to a wide range of soils
1
1985
... Feest & Madelin(1985)最早通过计数培养后获得的黏菌原生质团定量分析了土壤中分布的黏变形体,研究结果表明,黏菌的黏变形体普遍栖生于森林、草原和农田等不同类型的土壤中,丰度约为8 000个/cm3,其季节动态与细菌丰富相关(Feest & Madelin 1988).在采用分子生物学方法开展的研究中,Kamono & Fukui(2006)将PCR-DGGE技术应用于分析土壤绒泡菌目Physarales的营养体群落,发现不同深度土层的黏菌种类存在差异,但未检测到明显的季节动态变化(Kamono et al. 2009b).在后续研究中,Kamono et al.(2013)以SSU rRNA基因扩增子克隆测序的方法分析了雪线土壤中的暗孢黏菌多样性,序列注释结果表明,亮皮菌属Lamproderma、双皮菌属Diderma和钙皮菌属Didymium为优势类群,来自法国、苏格兰和日本的土壤样品中,黏菌物种组成显著不同.在腐木中,Clissmann et al.(2015)获得29种亮孢黏菌的可操作分类单元(operational taxa units,OTUs),序列注释结果表明,团网菌属Arcyria、团毛菌属Trichia和粉瘤菌属Lycogala是优势属,腐木的pH是决定黏菌群落分布的主要环境因子. ...
Seasonal population changes of myxomycetes and associated organisms in four woodland soils
1
1988
... Feest & Madelin(1985)最早通过计数培养后获得的黏菌原生质团定量分析了土壤中分布的黏变形体,研究结果表明,黏菌的黏变形体普遍栖生于森林、草原和农田等不同类型的土壤中,丰度约为8 000个/cm3,其季节动态与细菌丰富相关(Feest & Madelin 1988).在采用分子生物学方法开展的研究中,Kamono & Fukui(2006)将PCR-DGGE技术应用于分析土壤绒泡菌目Physarales的营养体群落,发现不同深度土层的黏菌种类存在差异,但未检测到明显的季节动态变化(Kamono et al. 2009b).在后续研究中,Kamono et al.(2013)以SSU rRNA基因扩增子克隆测序的方法分析了雪线土壤中的暗孢黏菌多样性,序列注释结果表明,亮皮菌属Lamproderma、双皮菌属Diderma和钙皮菌属Didymium为优势类群,来自法国、苏格兰和日本的土壤样品中,黏菌物种组成显著不同.在腐木中,Clissmann et al.(2015)获得29种亮孢黏菌的可操作分类单元(operational taxa units,OTUs),序列注释结果表明,团网菌属Arcyria、团毛菌属Trichia和粉瘤菌属Lycogala是优势属,腐木的pH是决定黏菌群落分布的主要环境因子. ...
What an intron may tell: several sexual biospecies coexist in Meriderma spp. (Myxomycetes)
1
2016
... 黏菌形态种下可能存在多个隐存种,这些隐存种之间彼此存在生殖隔离,但难以从形态上区分(Feng & Schnittler 2015;Feng et al. 2016),进而干扰所研究物种的地理分布模式.目前,在许多黏菌的生物地理分布研究中增加了分子标记手段,通过基因型的差异更准确地反映物种分布,但结论也不尽相同.Winsett & Stephenson(2011)以mt SSU rRNA基因分析了畸形钙皮菌Didymium difforme的地理分布规律,13种mt SSU rRNA基因型为随机分布.Hoppe(2013)研究显示煤绒菌黄色变种Fuligo septica var. flava可根据mt SSU rRNA基因分为3个分支,分支间的遗传距离与地理距离不相关.Aguilar et al.(2014)采用SSU rRNA基因系统发育分析和环境生态位模拟,分析了美洲的暗孢钙丝菌Badhamia melanospora种群遗传分化,结果显示,南北美洲的暗孢钙丝菌B. melanospora按照大陆的分离完全隔断为南北两个基因型的种群,大部分种群局限分布在一个较为固定的地理范围内.Dagamac et al.(2017c)通过SSU rRNA基因序列分析发现蛇形半网菌Hemitrichia serpula包含3个主要的系统发育群,不同种群的地理分布、形态特征和EF-1α基因序列也显著不同.Janik et al.(2020)研究了雪生钙皮菌Didymium nivicola的SSU rRNA和EF-1α基因多态性,发现在安第斯山脉的种内遗传多样性最高,包含17种SSU核糖体型和12种EF-1α基因型,而北半球种群的遗传背景则比较一致,因此认为安第斯山脉可能是雪生钙皮菌D. nivicola的遗传多样性分化中心. ...
Sex or no sex? Group I introns and independent marker genes reveal the existence of three sexual but reproductively isolated biospecies in Trichia varia (Myxomycetes)
1
2015
... 黏菌形态种下可能存在多个隐存种,这些隐存种之间彼此存在生殖隔离,但难以从形态上区分(Feng & Schnittler 2015;Feng et al. 2016),进而干扰所研究物种的地理分布模式.目前,在许多黏菌的生物地理分布研究中增加了分子标记手段,通过基因型的差异更准确地反映物种分布,但结论也不尽相同.Winsett & Stephenson(2011)以mt SSU rRNA基因分析了畸形钙皮菌Didymium difforme的地理分布规律,13种mt SSU rRNA基因型为随机分布.Hoppe(2013)研究显示煤绒菌黄色变种Fuligo septica var. flava可根据mt SSU rRNA基因分为3个分支,分支间的遗传距离与地理距离不相关.Aguilar et al.(2014)采用SSU rRNA基因系统发育分析和环境生态位模拟,分析了美洲的暗孢钙丝菌Badhamia melanospora种群遗传分化,结果显示,南北美洲的暗孢钙丝菌B. melanospora按照大陆的分离完全隔断为南北两个基因型的种群,大部分种群局限分布在一个较为固定的地理范围内.Dagamac et al.(2017c)通过SSU rRNA基因序列分析发现蛇形半网菌Hemitrichia serpula包含3个主要的系统发育群,不同种群的地理分布、形态特征和EF-1α基因序列也显著不同.Janik et al.(2020)研究了雪生钙皮菌Didymium nivicola的SSU rRNA和EF-1α基因多态性,发现在安第斯山脉的种内遗传多样性最高,包含17种SSU核糖体型和12种EF-1α基因型,而北半球种群的遗传背景则比较一致,因此认为安第斯山脉可能是雪生钙皮菌D. nivicola的遗传多样性分化中心. ...
Global dispersal of free-living microbial eukaryote species
1
2002
... 原生动物的生物地理分布模式,存在两种假说:一为原生动物在全球范围内呈随机分布,其分布主要由所处生境中的环境因子决定,即“everything is everywhere, but the environment selects”(Finlay 2002);二为与之相对应的地带性分布(moderate endemicity),指物种的分布受扩散限制的影响(Foissner 2006).黏菌归属于原生动物界已经获得普遍接受(Alexopoulos et al. 1996),其孢子能够借助气流进行长距离散播(Kamono et al. 2009a),具有较强的扩散能力.基于标本采集的物种调查和形态分类学研究结果一方面为黏菌的随机分布提供了证据支持,如许多黏菌物种呈现出跨大陆的分布格局(Stephenson et al. 2008).黏菌物种调查研究同时显示,偏远海岛的物种组成与大陆类似而无特有种存在,包括美国夏威夷群岛(Eliasson 1991)和澳大利亚麦夸里岛(Stephenson et al. 2007)等.另一方面,一些研究结果表明黏菌也可能受扩散限制的影响,而呈现地带性分布,如裂瓣菌Barbeyella minutissima集中分布于温带森林,而圆孢鹅绒菌Ceratiomyxa sphaerosperma仅知分布于美洲的热带地区(Stephenson & Rojas 2017).Dagamac et al.(2017b)研究了新热带和古热带地区两种森林生态系统的黏菌群落组成,发现一些物种的分布局限于特定的地理区域,地理距离而非生境差异对黏菌群落组成影响更大. ...
Metacommunity analysis of amoeboid protists in grassland soils
1
2016
... 高通量测序技术的应用极大地增加了扩增子测序的测序深度,目前从草原(Fiore- Donno et al. 2016)、融雪带(Borg Dahl et al. 2018b)和森林(Borg Dahl et al. 2019;Gao et al. 2019;Shchepin et al. 2019)土壤中所报道的暗孢黏菌OTUs数量在200-300之间.在群落组成方面,已有18属暗孢黏菌报道分布于土壤中,其中亮皮菌属Lamproderma、发网菌属Stemonitis、裂皮菌属Meriderma和双皮菌属Diderma为优势属,一些典型的林中凋落物栖生黏菌如高杯菌Craterium minutum、光果菌Leocarpus fragilis等也被发现分布于土壤中(Gao et al. 2019;Shchepin et al. 2019).土壤中黏菌的分布格局也得到进一步认识,例如,森林火灾后不同年份土壤的黏菌组成显著不同,表明黏菌群落存在显著的演替规律;不同植被土壤的黏菌群落存在显著差异,并主要由土壤pH驱动(Borg Dahl et al. 2019;Gao et al. 2019).我们整合了目前已发表的土壤暗孢黏菌高通量测序数据(PRJNA418896;PRJNA491744;PRJNA565474),通过非度量多维尺度(non-metric multidimensional scaling,NMDS)分析发现,德国阿尔卑斯山地森林、俄罗斯西北部泰加林和中国河南宝天曼保护区温带森林的土壤黏菌群落组成显著不同(图2),这也为黏菌的大尺度地带性分布格局提供了佐证. ...
Biogeography and dispersal of micro-organisms: a review emphasizing protists
1
2006
... 原生动物的生物地理分布模式,存在两种假说:一为原生动物在全球范围内呈随机分布,其分布主要由所处生境中的环境因子决定,即“everything is everywhere, but the environment selects”(Finlay 2002);二为与之相对应的地带性分布(moderate endemicity),指物种的分布受扩散限制的影响(Foissner 2006).黏菌归属于原生动物界已经获得普遍接受(Alexopoulos et al. 1996),其孢子能够借助气流进行长距离散播(Kamono et al. 2009a),具有较强的扩散能力.基于标本采集的物种调查和形态分类学研究结果一方面为黏菌的随机分布提供了证据支持,如许多黏菌物种呈现出跨大陆的分布格局(Stephenson et al. 2008).黏菌物种调查研究同时显示,偏远海岛的物种组成与大陆类似而无特有种存在,包括美国夏威夷群岛(Eliasson 1991)和澳大利亚麦夸里岛(Stephenson et al. 2007)等.另一方面,一些研究结果表明黏菌也可能受扩散限制的影响,而呈现地带性分布,如裂瓣菌Barbeyella minutissima集中分布于温带森林,而圆孢鹅绒菌Ceratiomyxa sphaerosperma仅知分布于美洲的热带地区(Stephenson & Rojas 2017).Dagamac et al.(2017b)研究了新热带和古热带地区两种森林生态系统的黏菌群落组成,发现一些物种的分布局限于特定的地理区域,地理距离而非生境差异对黏菌群落组成影响更大. ...
Nutrient mobilization by plasmodium of myxomycete Physarum rigidum in deadwood
0
2017
Myxomycete diversity and ecology in the Baotianman National Nature Reserve, a subtropical mountain forest in central China
2
2018
... 热带、亚热带黏菌的早期研究仅限于零星报道.20世纪70年代,Farr(1976)系统整理了前人文献资料并记述了新热带区280种黏菌.在此基础上,Lado & Wrigley de Basanta(2008)重新梳理了分布于新热带地区的黏菌物种,共计431种.近年,研究人员陆续在我国热带和亚热带森林(闫淑珍等2012;Liu et al. 2013;魏滨等 2016;Gao et al. 2018;Li et al. 2021)、东南亚热带雨林开展了系统调查(Tran et al. 2006;2008;Ko Ko et al. 2010,2012,2013;Dagamac et al. 2011,2014,2017a;Novozhilov et al. 2017a,2020),极大地丰富了黏菌物种的地理分布和区系特征. ...
... 黏菌群落的时间动态一般由不同季节的湿热条件决定(Stephenson 1988;Takahashi & Hada 2012).基于野外采集和湿室培养研究热带地区的低海拔森林和农田生态系统发现,雨季黏菌的丰富度和多样性显著高于旱季(Tran et al. 2006;2008;Ko Ko et al. 2011).宋天鹏和陈双林(2014)在中国云南黄连山保护区较高海拔的亚热带森林的研究显示,雨季的黏菌多样性低于旱季,该研究通过湿室培养法收集黏菌子实体,反映出旱季的基物上存在更多黏菌休眠体.一些黏菌的发生具有季节性,造就了黏菌群落组成的季节性差异,如发网菌目黏菌多发生于雨季、绒泡菌目多发生于旱季(Ko Ko et al. 2011).日本温带森林凋落物栖生黏菌具有明显的季节发生规律,根据物种组成分为早期(4-5月)、中期(6-8月)和晚期(10-11月)3个阶段(Takahashi & Hada 2012).类似地,中国河南宝天曼保护区林中凋落物栖生黏菌群落在春、夏、秋3季也显著不同,基物类型及月平均气温与降水等气象因子是影响黏菌群落组成的主要因素(Gao et al. 2018). ...
Influence of forest type on dark-spored myxomycete community in subtropical forest soil, China
3
2019
... 高通量测序技术的应用极大地增加了扩增子测序的测序深度,目前从草原(Fiore- Donno et al. 2016)、融雪带(Borg Dahl et al. 2018b)和森林(Borg Dahl et al. 2019;Gao et al. 2019;Shchepin et al. 2019)土壤中所报道的暗孢黏菌OTUs数量在200-300之间.在群落组成方面,已有18属暗孢黏菌报道分布于土壤中,其中亮皮菌属Lamproderma、发网菌属Stemonitis、裂皮菌属Meriderma和双皮菌属Diderma为优势属,一些典型的林中凋落物栖生黏菌如高杯菌Craterium minutum、光果菌Leocarpus fragilis等也被发现分布于土壤中(Gao et al. 2019;Shchepin et al. 2019).土壤中黏菌的分布格局也得到进一步认识,例如,森林火灾后不同年份土壤的黏菌组成显著不同,表明黏菌群落存在显著的演替规律;不同植被土壤的黏菌群落存在显著差异,并主要由土壤pH驱动(Borg Dahl et al. 2019;Gao et al. 2019).我们整合了目前已发表的土壤暗孢黏菌高通量测序数据(PRJNA418896;PRJNA491744;PRJNA565474),通过非度量多维尺度(non-metric multidimensional scaling,NMDS)分析发现,德国阿尔卑斯山地森林、俄罗斯西北部泰加林和中国河南宝天曼保护区温带森林的土壤黏菌群落组成显著不同(图2),这也为黏菌的大尺度地带性分布格局提供了佐证. ...
... 等也被发现分布于土壤中(Gao et al. 2019;Shchepin et al. 2019).土壤中黏菌的分布格局也得到进一步认识,例如,森林火灾后不同年份土壤的黏菌组成显著不同,表明黏菌群落存在显著的演替规律;不同植被土壤的黏菌群落存在显著差异,并主要由土壤pH驱动(Borg Dahl et al. 2019;Gao et al. 2019).我们整合了目前已发表的土壤暗孢黏菌高通量测序数据(PRJNA418896;PRJNA491744;PRJNA565474),通过非度量多维尺度(non-metric multidimensional scaling,NMDS)分析发现,德国阿尔卑斯山地森林、俄罗斯西北部泰加林和中国河南宝天曼保护区温带森林的土壤黏菌群落组成显著不同(图2),这也为黏菌的大尺度地带性分布格局提供了佐证. ...
... ;Gao et al. 2019).我们整合了目前已发表的土壤暗孢黏菌高通量测序数据(PRJNA418896;PRJNA491744;PRJNA565474),通过非度量多维尺度(non-metric multidimensional scaling,NMDS)分析发现,德国阿尔卑斯山地森林、俄罗斯西北部泰加林和中国河南宝天曼保护区温带森林的土壤黏菌群落组成显著不同(图2),这也为黏菌的大尺度地带性分布格局提供了佐证. ...
Protists as catalyzers of microbial litter breakdown and carbon cycling at different temperature regimes
1
2021
... 黏菌最常见于林下潮湿的凋落物、树皮、倒木、食草动物粪便和土壤甚至活的动物体中,能以伪足取食基质最细微孔隙中的细菌、真菌及其他微生物(Liu et al. 2015;李敏和陈双林 2021).黏菌的这一摄食活动可增加植物凋落物分解过程中的CO2释放速率和质量损失(Geisen et al. 2021),促进N、P、K等元素的循环(Fukasawa et al. 2017),是森林生态系统物质循环和能量流动过程中不可替代的参与者.黏菌的地理分布和生态学研究是当前黏菌生物学研究的重要领域之一,已有研究不同程度地揭示了黏菌的地理分布特征、生态位特征、时空分布格局以及与其他生物或非生物因子的关系等,对于理解黏菌的生态系统服务和功能具有重要作用.本文对黏菌分布格局和生态学研究中的进展性成果进行综述,并结合当前研究动态,对今后研究提出展望. ...
Molecular diversity of myxomycetes near Siegen (Germany)
1
2013
... 黏菌形态种下可能存在多个隐存种,这些隐存种之间彼此存在生殖隔离,但难以从形态上区分(Feng & Schnittler 2015;Feng et al. 2016),进而干扰所研究物种的地理分布模式.目前,在许多黏菌的生物地理分布研究中增加了分子标记手段,通过基因型的差异更准确地反映物种分布,但结论也不尽相同.Winsett & Stephenson(2011)以mt SSU rRNA基因分析了畸形钙皮菌Didymium difforme的地理分布规律,13种mt SSU rRNA基因型为随机分布.Hoppe(2013)研究显示煤绒菌黄色变种Fuligo septica var. flava可根据mt SSU rRNA基因分为3个分支,分支间的遗传距离与地理距离不相关.Aguilar et al.(2014)采用SSU rRNA基因系统发育分析和环境生态位模拟,分析了美洲的暗孢钙丝菌Badhamia melanospora种群遗传分化,结果显示,南北美洲的暗孢钙丝菌B. melanospora按照大陆的分离完全隔断为南北两个基因型的种群,大部分种群局限分布在一个较为固定的地理范围内.Dagamac et al.(2017c)通过SSU rRNA基因序列分析发现蛇形半网菌Hemitrichia serpula包含3个主要的系统发育群,不同种群的地理分布、形态特征和EF-1α基因序列也显著不同.Janik et al.(2020)研究了雪生钙皮菌Didymium nivicola的SSU rRNA和EF-1α基因多态性,发现在安第斯山脉的种内遗传多样性最高,包含17种SSU核糖体型和12种EF-1α基因型,而北半球种群的遗传背景则比较一致,因此认为安第斯山脉可能是雪生钙皮菌D. nivicola的遗传多样性分化中心. ...
Characterization of myxomycetes in two different soils by TRFLP-analysis of partial 18S rRNA gene sequences
1
2015
... 早期的黏菌生物学围绕黏菌生活史开展了大量研究,其中一些研究揭示了孢子萌发、黏变形体生长、原生质团行为等与所处的生态环境,包括温度、湿度、pH等一系列环境因素之间的关系(Madelin 1984),可以将这些研究归纳到个体生态学的范畴.Maimoni- Rodella & Gottsberger(1980)定量分析了巴西热带森林中的黏菌群落与基质类型、温度和降水量的关系,开启了黏菌的群落生态学研究.目前,绝大多数黏菌生态学研究首先通过子实体调查和分类鉴定,获得一定区域或生境内的黏菌物种组成与丰度信息;再应用数量生态学的分析手段,揭示黏菌群落的物种分布格局和影响因素等(Stephenson & Rojas 2017).这种基于子实体调查的方法不可避免地忽略了环境中一些无法发育为子实体的黏菌营养体(李玉等 2021),因此,许多研究者尝试将DNA分子标记和指纹图谱技术用于解析环境中的黏菌群落,如PCR-DGGE(Kamono & Fukui 2006;Kamono et al. 2009b)、PCR-T-RFLP(Hoppe & Schnittler 2015)等,标志着黏菌生态学研究已进入分子时代.当前,黏菌DNA条形码与高通量测序结合产生的扩增子高通量测序为黏菌生态学研究提供了新方法,通过环境基因组DNA提取、条形码基因PCR扩增、产物高通量测序和序列分类注释,已实现了不依赖子实体调查的群落定性、定量分析(Borg Dahl et al. 2018a). ...
Range-wide phylogeography of a nivicolous protist Didymium nivicola Meyl. (Myxomycetes, Amoebozoa): striking contrasts between the Northern and the Southern Hemisphere
1
2020
... 黏菌形态种下可能存在多个隐存种,这些隐存种之间彼此存在生殖隔离,但难以从形态上区分(Feng & Schnittler 2015;Feng et al. 2016),进而干扰所研究物种的地理分布模式.目前,在许多黏菌的生物地理分布研究中增加了分子标记手段,通过基因型的差异更准确地反映物种分布,但结论也不尽相同.Winsett & Stephenson(2011)以mt SSU rRNA基因分析了畸形钙皮菌Didymium difforme的地理分布规律,13种mt SSU rRNA基因型为随机分布.Hoppe(2013)研究显示煤绒菌黄色变种Fuligo septica var. flava可根据mt SSU rRNA基因分为3个分支,分支间的遗传距离与地理距离不相关.Aguilar et al.(2014)采用SSU rRNA基因系统发育分析和环境生态位模拟,分析了美洲的暗孢钙丝菌Badhamia melanospora种群遗传分化,结果显示,南北美洲的暗孢钙丝菌B. melanospora按照大陆的分离完全隔断为南北两个基因型的种群,大部分种群局限分布在一个较为固定的地理范围内.Dagamac et al.(2017c)通过SSU rRNA基因序列分析发现蛇形半网菌Hemitrichia serpula包含3个主要的系统发育群,不同种群的地理分布、形态特征和EF-1α基因序列也显著不同.Janik et al.(2020)研究了雪生钙皮菌Didymium nivicola的SSU rRNA和EF-1α基因多态性,发现在安第斯山脉的种内遗传多样性最高,包含17种SSU核糖体型和12种EF-1α基因型,而北半球种群的遗传背景则比较一致,因此认为安第斯山脉可能是雪生钙皮菌D. nivicola的遗传多样性分化中心. ...
Rapid PCR-based method for detection and differentiation of Didymiaceae and Physaraceae (myxomycetes) in environmental samples
2
2006
... 早期的黏菌生物学围绕黏菌生活史开展了大量研究,其中一些研究揭示了孢子萌发、黏变形体生长、原生质团行为等与所处的生态环境,包括温度、湿度、pH等一系列环境因素之间的关系(Madelin 1984),可以将这些研究归纳到个体生态学的范畴.Maimoni- Rodella & Gottsberger(1980)定量分析了巴西热带森林中的黏菌群落与基质类型、温度和降水量的关系,开启了黏菌的群落生态学研究.目前,绝大多数黏菌生态学研究首先通过子实体调查和分类鉴定,获得一定区域或生境内的黏菌物种组成与丰度信息;再应用数量生态学的分析手段,揭示黏菌群落的物种分布格局和影响因素等(Stephenson & Rojas 2017).这种基于子实体调查的方法不可避免地忽略了环境中一些无法发育为子实体的黏菌营养体(李玉等 2021),因此,许多研究者尝试将DNA分子标记和指纹图谱技术用于解析环境中的黏菌群落,如PCR-DGGE(Kamono & Fukui 2006;Kamono et al. 2009b)、PCR-T-RFLP(Hoppe & Schnittler 2015)等,标志着黏菌生态学研究已进入分子时代.当前,黏菌DNA条形码与高通量测序结合产生的扩增子高通量测序为黏菌生态学研究提供了新方法,通过环境基因组DNA提取、条形码基因PCR扩增、产物高通量测序和序列分类注释,已实现了不依赖子实体调查的群落定性、定量分析(Borg Dahl et al. 2018a). ...
... Feest & Madelin(1985)最早通过计数培养后获得的黏菌原生质团定量分析了土壤中分布的黏变形体,研究结果表明,黏菌的黏变形体普遍栖生于森林、草原和农田等不同类型的土壤中,丰度约为8 000个/cm3,其季节动态与细菌丰富相关(Feest & Madelin 1988).在采用分子生物学方法开展的研究中,Kamono & Fukui(2006)将PCR-DGGE技术应用于分析土壤绒泡菌目Physarales的营养体群落,发现不同深度土层的黏菌种类存在差异,但未检测到明显的季节动态变化(Kamono et al. 2009b).在后续研究中,Kamono et al.(2013)以SSU rRNA基因扩增子克隆测序的方法分析了雪线土壤中的暗孢黏菌多样性,序列注释结果表明,亮皮菌属Lamproderma、双皮菌属Diderma和钙皮菌属Didymium为优势类群,来自法国、苏格兰和日本的土壤样品中,黏菌物种组成显著不同.在腐木中,Clissmann et al.(2015)获得29种亮孢黏菌的可操作分类单元(operational taxa units,OTUs),序列注释结果表明,团网菌属Arcyria、团毛菌属Trichia和粉瘤菌属Lycogala是优势属,腐木的pH是决定黏菌群落分布的主要环境因子. ...
Airborne myxomycete spores: detection using molecular techniques
1
2009a
... 原生动物的生物地理分布模式,存在两种假说:一为原生动物在全球范围内呈随机分布,其分布主要由所处生境中的环境因子决定,即“everything is everywhere, but the environment selects”(Finlay 2002);二为与之相对应的地带性分布(moderate endemicity),指物种的分布受扩散限制的影响(Foissner 2006).黏菌归属于原生动物界已经获得普遍接受(Alexopoulos et al. 1996),其孢子能够借助气流进行长距离散播(Kamono et al. 2009a),具有较强的扩散能力.基于标本采集的物种调查和形态分类学研究结果一方面为黏菌的随机分布提供了证据支持,如许多黏菌物种呈现出跨大陆的分布格局(Stephenson et al. 2008).黏菌物种调查研究同时显示,偏远海岛的物种组成与大陆类似而无特有种存在,包括美国夏威夷群岛(Eliasson 1991)和澳大利亚麦夸里岛(Stephenson et al. 2007)等.另一方面,一些研究结果表明黏菌也可能受扩散限制的影响,而呈现地带性分布,如裂瓣菌Barbeyella minutissima集中分布于温带森林,而圆孢鹅绒菌Ceratiomyxa sphaerosperma仅知分布于美洲的热带地区(Stephenson & Rojas 2017).Dagamac et al.(2017b)研究了新热带和古热带地区两种森林生态系统的黏菌群落组成,发现一些物种的分布局限于特定的地理区域,地理距离而非生境差异对黏菌群落组成影响更大. ...
Characterization of myxomycete communities in soil by reverse transcription polymerase chain reaction (RT-PCR)-based method
2
2009b
... 早期的黏菌生物学围绕黏菌生活史开展了大量研究,其中一些研究揭示了孢子萌发、黏变形体生长、原生质团行为等与所处的生态环境,包括温度、湿度、pH等一系列环境因素之间的关系(Madelin 1984),可以将这些研究归纳到个体生态学的范畴.Maimoni- Rodella & Gottsberger(1980)定量分析了巴西热带森林中的黏菌群落与基质类型、温度和降水量的关系,开启了黏菌的群落生态学研究.目前,绝大多数黏菌生态学研究首先通过子实体调查和分类鉴定,获得一定区域或生境内的黏菌物种组成与丰度信息;再应用数量生态学的分析手段,揭示黏菌群落的物种分布格局和影响因素等(Stephenson & Rojas 2017).这种基于子实体调查的方法不可避免地忽略了环境中一些无法发育为子实体的黏菌营养体(李玉等 2021),因此,许多研究者尝试将DNA分子标记和指纹图谱技术用于解析环境中的黏菌群落,如PCR-DGGE(Kamono & Fukui 2006;Kamono et al. 2009b)、PCR-T-RFLP(Hoppe & Schnittler 2015)等,标志着黏菌生态学研究已进入分子时代.当前,黏菌DNA条形码与高通量测序结合产生的扩增子高通量测序为黏菌生态学研究提供了新方法,通过环境基因组DNA提取、条形码基因PCR扩增、产物高通量测序和序列分类注释,已实现了不依赖子实体调查的群落定性、定量分析(Borg Dahl et al. 2018a). ...
... Feest & Madelin(1985)最早通过计数培养后获得的黏菌原生质团定量分析了土壤中分布的黏变形体,研究结果表明,黏菌的黏变形体普遍栖生于森林、草原和农田等不同类型的土壤中,丰度约为8 000个/cm3,其季节动态与细菌丰富相关(Feest & Madelin 1988).在采用分子生物学方法开展的研究中,Kamono & Fukui(2006)将PCR-DGGE技术应用于分析土壤绒泡菌目Physarales的营养体群落,发现不同深度土层的黏菌种类存在差异,但未检测到明显的季节动态变化(Kamono et al. 2009b).在后续研究中,Kamono et al.(2013)以SSU rRNA基因扩增子克隆测序的方法分析了雪线土壤中的暗孢黏菌多样性,序列注释结果表明,亮皮菌属Lamproderma、双皮菌属Diderma和钙皮菌属Didymium为优势类群,来自法国、苏格兰和日本的土壤样品中,黏菌物种组成显著不同.在腐木中,Clissmann et al.(2015)获得29种亮孢黏菌的可操作分类单元(operational taxa units,OTUs),序列注释结果表明,团网菌属Arcyria、团毛菌属Trichia和粉瘤菌属Lycogala是优势属,腐木的pH是决定黏菌群落分布的主要环境因子. ...
Exploring slime mould diversity in high-altitude forests and grasslands by environmental RNA analysis
1
2013
... Feest & Madelin(1985)最早通过计数培养后获得的黏菌原生质团定量分析了土壤中分布的黏变形体,研究结果表明,黏菌的黏变形体普遍栖生于森林、草原和农田等不同类型的土壤中,丰度约为8 000个/cm3,其季节动态与细菌丰富相关(Feest & Madelin 1988).在采用分子生物学方法开展的研究中,Kamono & Fukui(2006)将PCR-DGGE技术应用于分析土壤绒泡菌目Physarales的营养体群落,发现不同深度土层的黏菌种类存在差异,但未检测到明显的季节动态变化(Kamono et al. 2009b).在后续研究中,Kamono et al.(2013)以SSU rRNA基因扩增子克隆测序的方法分析了雪线土壤中的暗孢黏菌多样性,序列注释结果表明,亮皮菌属Lamproderma、双皮菌属Diderma和钙皮菌属Didymium为优势类群,来自法国、苏格兰和日本的土壤样品中,黏菌物种组成显著不同.在腐木中,Clissmann et al.(2015)获得29种亮孢黏菌的可操作分类单元(operational taxa units,OTUs),序列注释结果表明,团网菌属Arcyria、团毛菌属Trichia和粉瘤菌属Lycogala是优势属,腐木的pH是决定黏菌群落分布的主要环境因子. ...
Myxomycetes of Myanmar
1
2013
... 热带、亚热带黏菌的早期研究仅限于零星报道.20世纪70年代,Farr(1976)系统整理了前人文献资料并记述了新热带区280种黏菌.在此基础上,Lado & Wrigley de Basanta(2008)重新梳理了分布于新热带地区的黏菌物种,共计431种.近年,研究人员陆续在我国热带和亚热带森林(闫淑珍等2012;Liu et al. 2013;魏滨等 2016;Gao et al. 2018;Li et al. 2021)、东南亚热带雨林开展了系统调查(Tran et al. 2006;2008;Ko Ko et al. 2010,2012,2013;Dagamac et al. 2011,2014,2017a;Novozhilov et al. 2017a,2020),极大地丰富了黏菌物种的地理分布和区系特征. ...
First records of myxomycetes in Laos
1
2012
... 热带、亚热带黏菌的早期研究仅限于零星报道.20世纪70年代,Farr(1976)系统整理了前人文献资料并记述了新热带区280种黏菌.在此基础上,Lado & Wrigley de Basanta(2008)重新梳理了分布于新热带地区的黏菌物种,共计431种.近年,研究人员陆续在我国热带和亚热带森林(闫淑珍等2012;Liu et al. 2013;魏滨等 2016;Gao et al. 2018;Li et al. 2021)、东南亚热带雨林开展了系统调查(Tran et al. 2006;2008;Ko Ko et al. 2010,2012,2013;Dagamac et al. 2011,2014,2017a;Novozhilov et al. 2017a,2020),极大地丰富了黏菌物种的地理分布和区系特征. ...
Myxomycetes of Thailand
1
2010
... 热带、亚热带黏菌的早期研究仅限于零星报道.20世纪70年代,Farr(1976)系统整理了前人文献资料并记述了新热带区280种黏菌.在此基础上,Lado & Wrigley de Basanta(2008)重新梳理了分布于新热带地区的黏菌物种,共计431种.近年,研究人员陆续在我国热带和亚热带森林(闫淑珍等2012;Liu et al. 2013;魏滨等 2016;Gao et al. 2018;Li et al. 2021)、东南亚热带雨林开展了系统调查(Tran et al. 2006;2008;Ko Ko et al. 2010,2012,2013;Dagamac et al. 2011,2014,2017a;Novozhilov et al. 2017a,2020),极大地丰富了黏菌物种的地理分布和区系特征. ...
Influence of seasonality on the occurrence of myxomycetes
2
2011
... 黏菌群落的时间动态一般由不同季节的湿热条件决定(Stephenson 1988;Takahashi & Hada 2012).基于野外采集和湿室培养研究热带地区的低海拔森林和农田生态系统发现,雨季黏菌的丰富度和多样性显著高于旱季(Tran et al. 2006;2008;Ko Ko et al. 2011).宋天鹏和陈双林(2014)在中国云南黄连山保护区较高海拔的亚热带森林的研究显示,雨季的黏菌多样性低于旱季,该研究通过湿室培养法收集黏菌子实体,反映出旱季的基物上存在更多黏菌休眠体.一些黏菌的发生具有季节性,造就了黏菌群落组成的季节性差异,如发网菌目黏菌多发生于雨季、绒泡菌目多发生于旱季(Ko Ko et al. 2011).日本温带森林凋落物栖生黏菌具有明显的季节发生规律,根据物种组成分为早期(4-5月)、中期(6-8月)和晚期(10-11月)3个阶段(Takahashi & Hada 2012).类似地,中国河南宝天曼保护区林中凋落物栖生黏菌群落在春、夏、秋3季也显著不同,基物类型及月平均气温与降水等气象因子是影响黏菌群落组成的主要因素(Gao et al. 2018). ...
... 在中国云南黄连山保护区较高海拔的亚热带森林的研究显示,雨季的黏菌多样性低于旱季,该研究通过湿室培养法收集黏菌子实体,反映出旱季的基物上存在更多黏菌休眠体.一些黏菌的发生具有季节性,造就了黏菌群落组成的季节性差异,如发网菌目黏菌多发生于雨季、绒泡菌目多发生于旱季(Ko Ko et al. 2011).日本温带森林凋落物栖生黏菌具有明显的季节发生规律,根据物种组成分为早期(4-5月)、中期(6-8月)和晚期(10-11月)3个阶段(Takahashi & Hada 2012).类似地,中国河南宝天曼保护区林中凋落物栖生黏菌群落在春、夏、秋3季也显著不同,基物类型及月平均气温与降水等气象因子是影响黏菌群落组成的主要因素(Gao et al. 2018). ...
1
2001
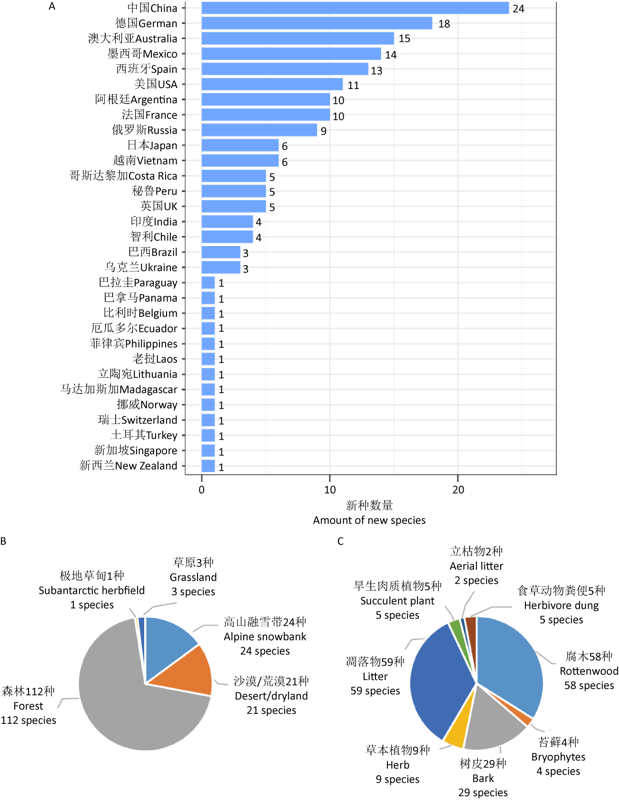
... 随着调查区域的拓展,在世界范围内发现并报道了一大批新分类单元,其中,仅2001-2020年的20年间,就有179个新种发表(Lado 2005-2021).上述新种及模式标本产地分布见图1A,其中描述自我国的新种最多,为24种;其次为德国18种;澳大利亚、墨西哥、西班牙、美国、阿根廷、法国和俄罗斯也有较多新种报道.从发现这些新种的生态系统来看(图1B),约70%来自森林生态系统,而干旱地区和高山融雪带也是新种发现的主要生境.另外,绝大多数新种采集于腐木(34.9%)、树皮(17.5%)和凋落物(35.5%)3种基质,相比之下,立枯物、食草动物粪便等一些特殊基质则有待进一步的深入调查研究(图1C). ...
Nivicolous myxomycetes from the Pyrenees: notes on the taxonomy and species diversity. Part 1. Physarales and Trichiales
1
2008
... 2003年,随着“全球黏菌生物多样性”(PBI: Global Biodiversity of Eumycetozoans)项目的实施,黏菌分类和多样性研究也拓展至中亚和南美沙漠.出乎意料的是,相关研究借助湿室培养技术,揭示出这一生态系统中具有极高的黏菌物种多样性(Novozhilov et al. 2006;Novozhilov & Schnittler 2008;Estrada-Torres et al. 2009;Lado et al. 2011;2013;2014;Schnittler et al. 2013;Wrigley de Basanta et al. 2013).另外,一些特殊生境中分布的黏菌也引起广泛关注,如发生于春季融雪带的雪线黏菌陆续报道于西班牙格雷多山(Lado et al. 2005)、比利牛斯山(Lado & Ronikier 2008,2009)、波兰喀尔巴阡山(Ronikier et al. 2008)、澳大利亚科西阿斯科山(Stephenson & Shadwick 2009)和俄罗斯高加索山(Novozhilov et al. 2012)等山脉.在极地生态系统开展的黏菌调查研究表明,阿拉斯加、冰岛、格陵兰岛和俄罗斯等北极地区广泛分布着北温带的稀有种(Stephenson et al. 2000);类似地,亚南极地区麦夸里岛也发现了一些罕见种(Wrigley de Basanta et al. 2010),表明极地生态系统拥有较高的黏菌物种特有性. ...
Nivicolous myxomycetes from the Pyrenees: notes on the taxonomy and species diversity. Part 2. Stemonitales
1
2009
... 2003年,随着“全球黏菌生物多样性”(PBI: Global Biodiversity of Eumycetozoans)项目的实施,黏菌分类和多样性研究也拓展至中亚和南美沙漠.出乎意料的是,相关研究借助湿室培养技术,揭示出这一生态系统中具有极高的黏菌物种多样性(Novozhilov et al. 2006;Novozhilov & Schnittler 2008;Estrada-Torres et al. 2009;Lado et al. 2011;2013;2014;Schnittler et al. 2013;Wrigley de Basanta et al. 2013).另外,一些特殊生境中分布的黏菌也引起广泛关注,如发生于春季融雪带的雪线黏菌陆续报道于西班牙格雷多山(Lado et al. 2005)、比利牛斯山(Lado & Ronikier 2008,2009)、波兰喀尔巴阡山(Ronikier et al. 2008)、澳大利亚科西阿斯科山(Stephenson & Shadwick 2009)和俄罗斯高加索山(Novozhilov et al. 2012)等山脉.在极地生态系统开展的黏菌调查研究表明,阿拉斯加、冰岛、格陵兰岛和俄罗斯等北极地区广泛分布着北温带的稀有种(Stephenson et al. 2000);类似地,亚南极地区麦夸里岛也发现了一些罕见种(Wrigley de Basanta et al. 2010),表明极地生态系统拥有较高的黏菌物种特有性. ...
Nivicolous myxomycetes from the Sierra de Gredos (central Spain)
1
2005
... 2003年,随着“全球黏菌生物多样性”(PBI: Global Biodiversity of Eumycetozoans)项目的实施,黏菌分类和多样性研究也拓展至中亚和南美沙漠.出乎意料的是,相关研究借助湿室培养技术,揭示出这一生态系统中具有极高的黏菌物种多样性(Novozhilov et al. 2006;Novozhilov & Schnittler 2008;Estrada-Torres et al. 2009;Lado et al. 2011;2013;2014;Schnittler et al. 2013;Wrigley de Basanta et al. 2013).另外,一些特殊生境中分布的黏菌也引起广泛关注,如发生于春季融雪带的雪线黏菌陆续报道于西班牙格雷多山(Lado et al. 2005)、比利牛斯山(Lado & Ronikier 2008,2009)、波兰喀尔巴阡山(Ronikier et al. 2008)、澳大利亚科西阿斯科山(Stephenson & Shadwick 2009)和俄罗斯高加索山(Novozhilov et al. 2012)等山脉.在极地生态系统开展的黏菌调查研究表明,阿拉斯加、冰岛、格陵兰岛和俄罗斯等北极地区广泛分布着北温带的稀有种(Stephenson et al. 2000);类似地,亚南极地区麦夸里岛也发现了一些罕见种(Wrigley de Basanta et al. 2010),表明极地生态系统拥有较高的黏菌物种特有性. ...
A review of neotropical Myxomycetes (1828-2008)
1
2008
... 热带、亚热带黏菌的早期研究仅限于零星报道.20世纪70年代,Farr(1976)系统整理了前人文献资料并记述了新热带区280种黏菌.在此基础上,Lado & Wrigley de Basanta(2008)重新梳理了分布于新热带地区的黏菌物种,共计431种.近年,研究人员陆续在我国热带和亚热带森林(闫淑珍等2012;Liu et al. 2013;魏滨等 2016;Gao et al. 2018;Li et al. 2021)、东南亚热带雨林开展了系统调查(Tran et al. 2006;2008;Ko Ko et al. 2010,2012,2013;Dagamac et al. 2011,2014,2017a;Novozhilov et al. 2017a,2020),极大地丰富了黏菌物种的地理分布和区系特征. ...
Biodiversity of Myxomycetes from the Monte desert of Argentina
1
2011
... 2003年,随着“全球黏菌生物多样性”(PBI: Global Biodiversity of Eumycetozoans)项目的实施,黏菌分类和多样性研究也拓展至中亚和南美沙漠.出乎意料的是,相关研究借助湿室培养技术,揭示出这一生态系统中具有极高的黏菌物种多样性(Novozhilov et al. 2006;Novozhilov & Schnittler 2008;Estrada-Torres et al. 2009;Lado et al. 2011;2013;2014;Schnittler et al. 2013;Wrigley de Basanta et al. 2013).另外,一些特殊生境中分布的黏菌也引起广泛关注,如发生于春季融雪带的雪线黏菌陆续报道于西班牙格雷多山(Lado et al. 2005)、比利牛斯山(Lado & Ronikier 2008,2009)、波兰喀尔巴阡山(Ronikier et al. 2008)、澳大利亚科西阿斯科山(Stephenson & Shadwick 2009)和俄罗斯高加索山(Novozhilov et al. 2012)等山脉.在极地生态系统开展的黏菌调查研究表明,阿拉斯加、冰岛、格陵兰岛和俄罗斯等北极地区广泛分布着北温带的稀有种(Stephenson et al. 2000);类似地,亚南极地区麦夸里岛也发现了一些罕见种(Wrigley de Basanta et al. 2010),表明极地生态系统拥有较高的黏菌物种特有性. ...
Myxomycete diversity of the Patagonian Steppe and bordering areas in Argentina
1
2014
... 2003年,随着“全球黏菌生物多样性”(PBI: Global Biodiversity of Eumycetozoans)项目的实施,黏菌分类和多样性研究也拓展至中亚和南美沙漠.出乎意料的是,相关研究借助湿室培养技术,揭示出这一生态系统中具有极高的黏菌物种多样性(Novozhilov et al. 2006;Novozhilov & Schnittler 2008;Estrada-Torres et al. 2009;Lado et al. 2011;2013;2014;Schnittler et al. 2013;Wrigley de Basanta et al. 2013).另外,一些特殊生境中分布的黏菌也引起广泛关注,如发生于春季融雪带的雪线黏菌陆续报道于西班牙格雷多山(Lado et al. 2005)、比利牛斯山(Lado & Ronikier 2008,2009)、波兰喀尔巴阡山(Ronikier et al. 2008)、澳大利亚科西阿斯科山(Stephenson & Shadwick 2009)和俄罗斯高加索山(Novozhilov et al. 2012)等山脉.在极地生态系统开展的黏菌调查研究表明,阿拉斯加、冰岛、格陵兰岛和俄罗斯等北极地区广泛分布着北温带的稀有种(Stephenson et al. 2000);类似地,亚南极地区麦夸里岛也发现了一些罕见种(Wrigley de Basanta et al. 2010),表明极地生态系统拥有较高的黏菌物种特有性. ...
The biodiversity of myxomycetes in central Chile
1
2013
... 2003年,随着“全球黏菌生物多样性”(PBI: Global Biodiversity of Eumycetozoans)项目的实施,黏菌分类和多样性研究也拓展至中亚和南美沙漠.出乎意料的是,相关研究借助湿室培养技术,揭示出这一生态系统中具有极高的黏菌物种多样性(Novozhilov et al. 2006;Novozhilov & Schnittler 2008;Estrada-Torres et al. 2009;Lado et al. 2011;2013;2014;Schnittler et al. 2013;Wrigley de Basanta et al. 2013).另外,一些特殊生境中分布的黏菌也引起广泛关注,如发生于春季融雪带的雪线黏菌陆续报道于西班牙格雷多山(Lado et al. 2005)、比利牛斯山(Lado & Ronikier 2008,2009)、波兰喀尔巴阡山(Ronikier et al. 2008)、澳大利亚科西阿斯科山(Stephenson & Shadwick 2009)和俄罗斯高加索山(Novozhilov et al. 2012)等山脉.在极地生态系统开展的黏菌调查研究表明,阿拉斯加、冰岛、格陵兰岛和俄罗斯等北极地区广泛分布着北温带的稀有种(Stephenson et al. 2000);类似地,亚南极地区麦夸里岛也发现了一些罕见种(Wrigley de Basanta et al. 2010),表明极地生态系统拥有较高的黏菌物种特有性. ...
The niche concept revisited: mechanistic models and community context
1
1995
... 生态位是指物种利用各种资源环境的总和,是研究物种间相互作用、对环境的适应性以及解释物种间竞争和共存机制的重要理论(Leibold 1995).在对黏菌种群生态位的研究中,通常选择几个重要因子进行生态位分析,如海拔、气温、降水量和基质类型等(Stephenson & Rojas 2017).总体来看,黏菌生态位宽度与物种的丰富度并无显著相关性,多数黏菌种类的生态位宽度较大,也即对环境的适应能力较强,是“生态泛化者”,而少数黏菌种类趋向成为特化种(Stephenson 1988).不同生态系统类型或基质栖生黏菌生态位也各具特点,如森林生态系统中的黏菌生态位宽度较大(Stephenson 1988;Rojas & Stephenson 2007),砂岩隐花植物生态系统中的黏菌生态位宽度较小(Schnittler et al. 2010);腐木栖生黏菌生态位宽度大,凋落物栖生的黏菌生态宽度小(Schnittler 2001;Rojas et al. 2010). ...
Towards a phylogenetic classification of the Myxomycetes
3
2019
... 黏菌Myxogastrea是原生动物界Protozoa变形虫门Amoebozoa中物种数量最为庞大的一个单系类群(Leontyev et al. 2019).生活史兼具原生动物和真菌的共同特征,营养阶段为单核的黏变形体(或游动胞)和多核的原生质团,繁殖阶段形成内含单倍体孢子的子实体(李玉等 2008).黏菌分类鉴定主要依靠子实体的形态特征,如基质层有无、柄的形态与质地、囊轴形态、孢丝有无与形态、孢子大小与纹饰等(李玉等 2008;Leontyev et al. 2019).自17世纪中叶起,菌物学工作者陆续在世界范围内开展了一系列黏菌资源的调查工作,迄今全球已被描述并认可的黏菌约1 000种(Lado 2005-2021).传统的黏菌分类系统将黏菌纲分为3亚纲6目(李玉等 2008),即鹅绒菌亚纲Ceratiomyxomycetidae鹅绒菌目Ceratiomyxales,腹黏菌亚纲Myxogastromycetidae刺轴菌目Echinosteliales、无丝菌目Liceales、团毛菌目Trichiales和绒泡菌目Physarales,发网菌亚纲Stemonitomycetidae发网菌目Stemonitales.晚近,Leontyev et al.(2019)结合分子系统学和形态分类学研究对黏菌分类系统做出较大调整,在保留主要的固有分类单元并遵循系统发育单系性的原则下,在黏菌纲中排除鹅绒菌目Ceratiomyxales,并且基于浅色孢子分支成立浅孢黏菌亚纲Lucisporomycetidae,其下含有无丝菌目Liceales、团毛菌目Trichiales、筛菌目Cribrariales和孔膜菌目Reticulariales;以子实体具有囊轴成立囊轴黏菌亚纲Columellomycetidae,其下含有刺轴菌目Echinosteliales、绒泡菌目Physarales、发网菌目Stemonitales、碎皮菌目Clastodermatales和裂皮菌目Meridermatales. ...
... ;Leontyev et al. 2019).自17世纪中叶起,菌物学工作者陆续在世界范围内开展了一系列黏菌资源的调查工作,迄今全球已被描述并认可的黏菌约1 000种(Lado 2005-2021).传统的黏菌分类系统将黏菌纲分为3亚纲6目(李玉等 2008),即鹅绒菌亚纲Ceratiomyxomycetidae鹅绒菌目Ceratiomyxales,腹黏菌亚纲Myxogastromycetidae刺轴菌目Echinosteliales、无丝菌目Liceales、团毛菌目Trichiales和绒泡菌目Physarales,发网菌亚纲Stemonitomycetidae发网菌目Stemonitales.晚近,Leontyev et al.(2019)结合分子系统学和形态分类学研究对黏菌分类系统做出较大调整,在保留主要的固有分类单元并遵循系统发育单系性的原则下,在黏菌纲中排除鹅绒菌目Ceratiomyxales,并且基于浅色孢子分支成立浅孢黏菌亚纲Lucisporomycetidae,其下含有无丝菌目Liceales、团毛菌目Trichiales、筛菌目Cribrariales和孔膜菌目Reticulariales;以子实体具有囊轴成立囊轴黏菌亚纲Columellomycetidae,其下含有刺轴菌目Echinosteliales、绒泡菌目Physarales、发网菌目Stemonitales、碎皮菌目Clastodermatales和裂皮菌目Meridermatales. ...
... ),即鹅绒菌亚纲Ceratiomyxomycetidae鹅绒菌目Ceratiomyxales,腹黏菌亚纲Myxogastromycetidae刺轴菌目Echinosteliales、无丝菌目Liceales、团毛菌目Trichiales和绒泡菌目Physarales,发网菌亚纲Stemonitomycetidae发网菌目Stemonitales.晚近,Leontyev et al.(2019)结合分子系统学和形态分类学研究对黏菌分类系统做出较大调整,在保留主要的固有分类单元并遵循系统发育单系性的原则下,在黏菌纲中排除鹅绒菌目Ceratiomyxales,并且基于浅色孢子分支成立浅孢黏菌亚纲Lucisporomycetidae,其下含有无丝菌目Liceales、团毛菌目Trichiales、筛菌目Cribrariales和孔膜菌目Reticulariales;以子实体具有囊轴成立囊轴黏菌亚纲Columellomycetidae,其下含有刺轴菌目Echinosteliales、绒泡菌目Physarales、发网菌目Stemonitales、碎皮菌目Clastodermatales和裂皮菌目Meridermatales. ...
Research progress on species diversity of myxomycetes and its relationship with influencing factors
0
2021
Spatiotemporal distribution and dynamic changes of myxomycetes in subtropical forests of China
1
2021
... 热带、亚热带黏菌的早期研究仅限于零星报道.20世纪70年代,Farr(1976)系统整理了前人文献资料并记述了新热带区280种黏菌.在此基础上,Lado & Wrigley de Basanta(2008)重新梳理了分布于新热带地区的黏菌物种,共计431种.近年,研究人员陆续在我国热带和亚热带森林(闫淑珍等2012;Liu et al. 2013;魏滨等 2016;Gao et al. 2018;Li et al. 2021)、东南亚热带雨林开展了系统调查(Tran et al. 2006;2008;Ko Ko et al. 2010,2012,2013;Dagamac et al. 2011,2014,2017a;Novozhilov et al. 2017a,2020),极大地丰富了黏菌物种的地理分布和区系特征. ...
Flora fungorum sinicorum. Myxomycetes I, II
0
2008
Development of myxomycetology
0
2021
Species diversity of myxomycetes associated with different terrestrial ecosystems, substrata (microhabitats) and environmental factors
1
2015
... 黏菌最常见于林下潮湿的凋落物、树皮、倒木、食草动物粪便和土壤甚至活的动物体中,能以伪足取食基质最细微孔隙中的细菌、真菌及其他微生物(Liu et al. 2015;李敏和陈双林 2021).黏菌的这一摄食活动可增加植物凋落物分解过程中的CO2释放速率和质量损失(Geisen et al. 2021),促进N、P、K等元素的循环(Fukasawa et al. 2017),是森林生态系统物质循环和能量流动过程中不可替代的参与者.黏菌的地理分布和生态学研究是当前黏菌生物学研究的重要领域之一,已有研究不同程度地揭示了黏菌的地理分布特征、生态位特征、时空分布格局以及与其他生物或非生物因子的关系等,对于理解黏菌的生态系统服务和功能具有重要作用.本文对黏菌分布格局和生态学研究中的进展性成果进行综述,并结合当前研究动态,对今后研究提出展望. ...
Species diversity of corticolous myxomycetes in Tianmu Mountain National Nature Reserve,
1
2013
... 热带、亚热带黏菌的早期研究仅限于零星报道.20世纪70年代,Farr(1976)系统整理了前人文献资料并记述了新热带区280种黏菌.在此基础上,Lado & Wrigley de Basanta(2008)重新梳理了分布于新热带地区的黏菌物种,共计431种.近年,研究人员陆续在我国热带和亚热带森林(闫淑珍等2012;Liu et al. 2013;魏滨等 2016;Gao et al. 2018;Li et al. 2021)、东南亚热带雨林开展了系统调查(Tran et al. 2006;2008;Ko Ko et al. 2010,2012,2013;Dagamac et al. 2011,2014,2017a;Novozhilov et al. 2017a,2020),极大地丰富了黏菌物种的地理分布和区系特征. ...
Myxomycete data of ecological significance
1
1984
... 早期的黏菌生物学围绕黏菌生活史开展了大量研究,其中一些研究揭示了孢子萌发、黏变形体生长、原生质团行为等与所处的生态环境,包括温度、湿度、pH等一系列环境因素之间的关系(Madelin 1984),可以将这些研究归纳到个体生态学的范畴.Maimoni- Rodella & Gottsberger(1980)定量分析了巴西热带森林中的黏菌群落与基质类型、温度和降水量的关系,开启了黏菌的群落生态学研究.目前,绝大多数黏菌生态学研究首先通过子实体调查和分类鉴定,获得一定区域或生境内的黏菌物种组成与丰度信息;再应用数量生态学的分析手段,揭示黏菌群落的物种分布格局和影响因素等(Stephenson & Rojas 2017).这种基于子实体调查的方法不可避免地忽略了环境中一些无法发育为子实体的黏菌营养体(李玉等 2021),因此,许多研究者尝试将DNA分子标记和指纹图谱技术用于解析环境中的黏菌群落,如PCR-DGGE(Kamono & Fukui 2006;Kamono et al. 2009b)、PCR-T-RFLP(Hoppe & Schnittler 2015)等,标志着黏菌生态学研究已进入分子时代.当前,黏菌DNA条形码与高通量测序结合产生的扩增子高通量测序为黏菌生态学研究提供了新方法,通过环境基因组DNA提取、条形码基因PCR扩增、产物高通量测序和序列分类注释,已实现了不依赖子实体调查的群落定性、定量分析(Borg Dahl et al. 2018a). ...
Myxomycetes from the forest and the Cerrado vegetation in Botucatu, Brazil: a comparative ecological study
1
1980
... 早期的黏菌生物学围绕黏菌生活史开展了大量研究,其中一些研究揭示了孢子萌发、黏变形体生长、原生质团行为等与所处的生态环境,包括温度、湿度、pH等一系列环境因素之间的关系(Madelin 1984),可以将这些研究归纳到个体生态学的范畴.Maimoni- Rodella & Gottsberger(1980)定量分析了巴西热带森林中的黏菌群落与基质类型、温度和降水量的关系,开启了黏菌的群落生态学研究.目前,绝大多数黏菌生态学研究首先通过子实体调查和分类鉴定,获得一定区域或生境内的黏菌物种组成与丰度信息;再应用数量生态学的分析手段,揭示黏菌群落的物种分布格局和影响因素等(Stephenson & Rojas 2017).这种基于子实体调查的方法不可避免地忽略了环境中一些无法发育为子实体的黏菌营养体(李玉等 2021),因此,许多研究者尝试将DNA分子标记和指纹图谱技术用于解析环境中的黏菌群落,如PCR-DGGE(Kamono & Fukui 2006;Kamono et al. 2009b)、PCR-T-RFLP(Hoppe & Schnittler 2015)等,标志着黏菌生态学研究已进入分子时代.当前,黏菌DNA条形码与高通量测序结合产生的扩增子高通量测序为黏菌生态学研究提供了新方法,通过环境基因组DNA提取、条形码基因PCR扩增、产物高通量测序和序列分类注释,已实现了不依赖子实体调查的群落定性、定量分析(Borg Dahl et al. 2018a). ...
Myxomycetes associated with monsoon lowland tropical forests in southern Vietnam
1
2017a
... 热带、亚热带黏菌的早期研究仅限于零星报道.20世纪70年代,Farr(1976)系统整理了前人文献资料并记述了新热带区280种黏菌.在此基础上,Lado & Wrigley de Basanta(2008)重新梳理了分布于新热带地区的黏菌物种,共计431种.近年,研究人员陆续在我国热带和亚热带森林(闫淑珍等2012;Liu et al. 2013;魏滨等 2016;Gao et al. 2018;Li et al. 2021)、东南亚热带雨林开展了系统调查(Tran et al. 2006;2008;Ko Ko et al. 2010,2012,2013;Dagamac et al. 2011,2014,2017a;Novozhilov et al. 2017a,2020),极大地丰富了黏菌物种的地理分布和区系特征. ...
Myxomycete diversity and ecology in arid regions of the Great Lake Basin of western Mongolia
1
2008
... 2003年,随着“全球黏菌生物多样性”(PBI: Global Biodiversity of Eumycetozoans)项目的实施,黏菌分类和多样性研究也拓展至中亚和南美沙漠.出乎意料的是,相关研究借助湿室培养技术,揭示出这一生态系统中具有极高的黏菌物种多样性(Novozhilov et al. 2006;Novozhilov & Schnittler 2008;Estrada-Torres et al. 2009;Lado et al. 2011;2013;2014;Schnittler et al. 2013;Wrigley de Basanta et al. 2013).另外,一些特殊生境中分布的黏菌也引起广泛关注,如发生于春季融雪带的雪线黏菌陆续报道于西班牙格雷多山(Lado et al. 2005)、比利牛斯山(Lado & Ronikier 2008,2009)、波兰喀尔巴阡山(Ronikier et al. 2008)、澳大利亚科西阿斯科山(Stephenson & Shadwick 2009)和俄罗斯高加索山(Novozhilov et al. 2012)等山脉.在极地生态系统开展的黏菌调查研究表明,阿拉斯加、冰岛、格陵兰岛和俄罗斯等北极地区广泛分布着北温带的稀有种(Stephenson et al. 2000);类似地,亚南极地区麦夸里岛也发现了一些罕见种(Wrigley de Basanta et al. 2010),表明极地生态系统拥有较高的黏菌物种特有性. ...
Diversity of nivicolous myxomycetes of the Teberda State Biosphere Reserve (Northwestern Caucasus, Russia)
1
2012
... 2003年,随着“全球黏菌生物多样性”(PBI: Global Biodiversity of Eumycetozoans)项目的实施,黏菌分类和多样性研究也拓展至中亚和南美沙漠.出乎意料的是,相关研究借助湿室培养技术,揭示出这一生态系统中具有极高的黏菌物种多样性(Novozhilov et al. 2006;Novozhilov & Schnittler 2008;Estrada-Torres et al. 2009;Lado et al. 2011;2013;2014;Schnittler et al. 2013;Wrigley de Basanta et al. 2013).另外,一些特殊生境中分布的黏菌也引起广泛关注,如发生于春季融雪带的雪线黏菌陆续报道于西班牙格雷多山(Lado et al. 2005)、比利牛斯山(Lado & Ronikier 2008,2009)、波兰喀尔巴阡山(Ronikier et al. 2008)、澳大利亚科西阿斯科山(Stephenson & Shadwick 2009)和俄罗斯高加索山(Novozhilov et al. 2012)等山脉.在极地生态系统开展的黏菌调查研究表明,阿拉斯加、冰岛、格陵兰岛和俄罗斯等北极地区广泛分布着北温带的稀有种(Stephenson et al. 2000);类似地,亚南极地区麦夸里岛也发现了一些罕见种(Wrigley de Basanta et al. 2010),表明极地生态系统拥有较高的黏菌物种特有性. ...
Myxomycetes of the Sikhote-Alin State Nature Biosphere Reserve Far East, Russia
1
2017b
... 自Panckow在1654年首次记录并描述粉瘤菌Lycogala epidendrum开始,尤其是Rostafinski在1873年建立第一个黏菌现代分类系统以来(Alexopoulos et al. 1996),黏菌工作者陆续在世界各地开展了大量的标本采集和经典分类的研究工作,出版了众多专著.但在很长一段时期内,研究区域主要集中于北半球的温带地区,其中以北美、欧洲和亚洲的温带森林生态系统最为突出(Stephenson et al. 2008),不仅有众多的论文,而且有许多地区志,包括北美的《The North American slime-moulds》和《The Mycetozoa of North America》,欧洲的《The Myxomycetes of Great Britain》《A monograph of the Mycetozoa— Being a descriptive catalogue of the species in the herbarium of the British Museum》《Mycological flora of European and Asian Russia. The slime molds》《A guide to temperate Myxomycetes》《Die Myxomyceten Deutschlands und des angrenzenden Alpenraumes unter besonderer Berücksichtigung Österreichs》《The Myxomycetes of Britain and Ireland. An identification handbook》和《Les Myxomycètes》,亚洲的《The Myxomycetes of Japan》《The Myxomycetes of India》《Taxonomy of the Indian Myxomycetes》《The myxomycete biota of Japan》和《中国真菌志·黏菌卷》等等.从已有调查结果来看,温带落叶阔叶林可能是地球上黏菌物种多样性最高的生态系统,如美国大雾山国家公园报道黏菌168种(Stephenson et al. 2001);俄罗斯锡霍特-阿林保护区报道黏菌158种(Novozhilov et al. 2017b);中国小兴安岭地区报道黏菌152种(赵凤云等 2019). ...
Myxomycetes associated with mountain tropical forests of Bidoup Nui Ba and Chu Yang Sin national parks (Dalat Plateau, southern Vietnam)
1
2020
... 热带、亚热带黏菌的早期研究仅限于零星报道.20世纪70年代,Farr(1976)系统整理了前人文献资料并记述了新热带区280种黏菌.在此基础上,Lado & Wrigley de Basanta(2008)重新梳理了分布于新热带地区的黏菌物种,共计431种.近年,研究人员陆续在我国热带和亚热带森林(闫淑珍等2012;Liu et al. 2013;魏滨等 2016;Gao et al. 2018;Li et al. 2021)、东南亚热带雨林开展了系统调查(Tran et al. 2006;2008;Ko Ko et al. 2010,2012,2013;Dagamac et al. 2011,2014,2017a;Novozhilov et al. 2017a,2020),极大地丰富了黏菌物种的地理分布和区系特征. ...
Myxomycete diversity and ecology in the arid regions of the Lower Volga River Basin (Russia)
1
2006
... 2003年,随着“全球黏菌生物多样性”(PBI: Global Biodiversity of Eumycetozoans)项目的实施,黏菌分类和多样性研究也拓展至中亚和南美沙漠.出乎意料的是,相关研究借助湿室培养技术,揭示出这一生态系统中具有极高的黏菌物种多样性(Novozhilov et al. 2006;Novozhilov & Schnittler 2008;Estrada-Torres et al. 2009;Lado et al. 2011;2013;2014;Schnittler et al. 2013;Wrigley de Basanta et al. 2013).另外,一些特殊生境中分布的黏菌也引起广泛关注,如发生于春季融雪带的雪线黏菌陆续报道于西班牙格雷多山(Lado et al. 2005)、比利牛斯山(Lado & Ronikier 2008,2009)、波兰喀尔巴阡山(Ronikier et al. 2008)、澳大利亚科西阿斯科山(Stephenson & Shadwick 2009)和俄罗斯高加索山(Novozhilov et al. 2012)等山脉.在极地生态系统开展的黏菌调查研究表明,阿拉斯加、冰岛、格陵兰岛和俄罗斯等北极地区广泛分布着北温带的稀有种(Stephenson et al. 2000);类似地,亚南极地区麦夸里岛也发现了一些罕见种(Wrigley de Basanta et al. 2010),表明极地生态系统拥有较高的黏菌物种特有性. ...
Distribution and ecology of myxomycetes in the high-elevation oak forests of Cerro Bellavista, Costa Rica
3
2007
... 生态位是指物种利用各种资源环境的总和,是研究物种间相互作用、对环境的适应性以及解释物种间竞争和共存机制的重要理论(Leibold 1995).在对黏菌种群生态位的研究中,通常选择几个重要因子进行生态位分析,如海拔、气温、降水量和基质类型等(Stephenson & Rojas 2017).总体来看,黏菌生态位宽度与物种的丰富度并无显著相关性,多数黏菌种类的生态位宽度较大,也即对环境的适应能力较强,是“生态泛化者”,而少数黏菌种类趋向成为特化种(Stephenson 1988).不同生态系统类型或基质栖生黏菌生态位也各具特点,如森林生态系统中的黏菌生态位宽度较大(Stephenson 1988;Rojas & Stephenson 2007),砂岩隐花植物生态系统中的黏菌生态位宽度较小(Schnittler et al. 2010);腐木栖生黏菌生态位宽度大,凋落物栖生的黏菌生态宽度小(Schnittler 2001;Rojas et al. 2010). ...
... 生态位重叠分析表明,黏菌物种间普遍具有较高的生态位重叠值(Stephenson 1988;Rojas & Stephenson 2007;Schnittler et al. 2010),即物种对环境资源有着相似的需求.但相对而言,子实体小型且原生质团隐型或原始型的树皮栖生黏菌生态位重叠度高,种间联结度高,而显型原生质团的凋落物栖生黏菌重叠度低,存在明显的种间竞争(Schnittler 2001).此外,黏菌的属内种间物种的生态位重叠度与属间物种生态位重叠度并无显著差异,反映了亲缘关系较远的黏菌演化出类似的生态策略(Rojas & Stephenson 2007). ...
... ).此外,黏菌的属内种间物种的生态位重叠度与属间物种生态位重叠度并无显著差异,反映了亲缘关系较远的黏菌演化出类似的生态策略(Rojas & Stephenson 2007). ...
Macroecology of high-elevation myxomycete assemblages in the northern Neotropics
1
2011
... 黏菌的纬度分布格局研究表明,黏菌的物种丰富度与纬度呈正相关关系,林型、土壤理化性质和基质pH是这一分布格局的主要驱动因子(Rojas et al. 2011).同时,在温带与热带森林黏菌多样性的比较研究中,温带森林均高于热带森林,热带地区过高的降水量和林下快速降解的凋落物不利于黏菌子实体的形成,同时,封闭的森林冠层使气流减少也可能会阻碍黏菌孢子的扩散(Stephenson et al. 1993;Cruz et al. 2014). ...
Does elevation influence the distributional patterns of tropical myxomycetes? A case study in Costa Rica
1
2016
... 黏菌群落的垂直分布格局研究显示,黏菌的物种多样性与海拔总体呈负相关关系,但驱动这一分布格局的环境因子各不相同,例如,Stephenson et al.(2004)认为在厄瓜多尔热带云雾林中过高的湿度条件是高海拔样地黏菌多样性低的主要原因;Rojas & Stephenson(2007)研究发现pH是驱动可可岛黏菌多样性黏菌垂直分布的关键因素;魏滨等(2016)认为不同海拔梯度下的气流差异驱动了中国广东鼎湖山自然保护区黏菌垂直分布的形成;Rojas et al.(2016)选择植被类型均一的海拔梯度样带进行研究后发现,黏菌多样性和群落组成均与海拔不相关,认为黏菌的垂直分布格局应归因于不同海拔的植被差异. ...
Ecological patterns of Costa Rican myxomycetes
1
2010
... 生态位是指物种利用各种资源环境的总和,是研究物种间相互作用、对环境的适应性以及解释物种间竞争和共存机制的重要理论(Leibold 1995).在对黏菌种群生态位的研究中,通常选择几个重要因子进行生态位分析,如海拔、气温、降水量和基质类型等(Stephenson & Rojas 2017).总体来看,黏菌生态位宽度与物种的丰富度并无显著相关性,多数黏菌种类的生态位宽度较大,也即对环境的适应能力较强,是“生态泛化者”,而少数黏菌种类趋向成为特化种(Stephenson 1988).不同生态系统类型或基质栖生黏菌生态位也各具特点,如森林生态系统中的黏菌生态位宽度较大(Stephenson 1988;Rojas & Stephenson 2007),砂岩隐花植物生态系统中的黏菌生态位宽度较小(Schnittler et al. 2010);腐木栖生黏菌生态位宽度大,凋落物栖生的黏菌生态宽度小(Schnittler 2001;Rojas et al. 2010). ...
Diversity of nivicolous myxomycetes in the Gorce mountains-a low-elevation massif of the Western Carpathians
1
2008
... 2003年,随着“全球黏菌生物多样性”(PBI: Global Biodiversity of Eumycetozoans)项目的实施,黏菌分类和多样性研究也拓展至中亚和南美沙漠.出乎意料的是,相关研究借助湿室培养技术,揭示出这一生态系统中具有极高的黏菌物种多样性(Novozhilov et al. 2006;Novozhilov & Schnittler 2008;Estrada-Torres et al. 2009;Lado et al. 2011;2013;2014;Schnittler et al. 2013;Wrigley de Basanta et al. 2013).另外,一些特殊生境中分布的黏菌也引起广泛关注,如发生于春季融雪带的雪线黏菌陆续报道于西班牙格雷多山(Lado et al. 2005)、比利牛斯山(Lado & Ronikier 2008,2009)、波兰喀尔巴阡山(Ronikier et al. 2008)、澳大利亚科西阿斯科山(Stephenson & Shadwick 2009)和俄罗斯高加索山(Novozhilov et al. 2012)等山脉.在极地生态系统开展的黏菌调查研究表明,阿拉斯加、冰岛、格陵兰岛和俄罗斯等北极地区广泛分布着北温带的稀有种(Stephenson et al. 2000);类似地,亚南极地区麦夸里岛也发现了一些罕见种(Wrigley de Basanta et al. 2010),表明极地生态系统拥有较高的黏菌物种特有性. ...
Ecology of myxomycetes of a winter-cold desert in western Kazakhstan
2
2001
... 生态位是指物种利用各种资源环境的总和,是研究物种间相互作用、对环境的适应性以及解释物种间竞争和共存机制的重要理论(Leibold 1995).在对黏菌种群生态位的研究中,通常选择几个重要因子进行生态位分析,如海拔、气温、降水量和基质类型等(Stephenson & Rojas 2017).总体来看,黏菌生态位宽度与物种的丰富度并无显著相关性,多数黏菌种类的生态位宽度较大,也即对环境的适应能力较强,是“生态泛化者”,而少数黏菌种类趋向成为特化种(Stephenson 1988).不同生态系统类型或基质栖生黏菌生态位也各具特点,如森林生态系统中的黏菌生态位宽度较大(Stephenson 1988;Rojas & Stephenson 2007),砂岩隐花植物生态系统中的黏菌生态位宽度较小(Schnittler et al. 2010);腐木栖生黏菌生态位宽度大,凋落物栖生的黏菌生态宽度小(Schnittler 2001;Rojas et al. 2010). ...
... 生态位重叠分析表明,黏菌物种间普遍具有较高的生态位重叠值(Stephenson 1988;Rojas & Stephenson 2007;Schnittler et al. 2010),即物种对环境资源有着相似的需求.但相对而言,子实体小型且原生质团隐型或原始型的树皮栖生黏菌生态位重叠度高,种间联结度高,而显型原生质团的凋落物栖生黏菌重叠度低,存在明显的种间竞争(Schnittler 2001).此外,黏菌的属内种间物种的生态位重叠度与属间物种生态位重叠度并无显著差异,反映了亲缘关系较远的黏菌演化出类似的生态策略(Rojas & Stephenson 2007). ...
Myxomycete diversity in the Tarim basin and eastern Tian-Shan, Xinjiang Province, China
1
2013
... 2003年,随着“全球黏菌生物多样性”(PBI: Global Biodiversity of Eumycetozoans)项目的实施,黏菌分类和多样性研究也拓展至中亚和南美沙漠.出乎意料的是,相关研究借助湿室培养技术,揭示出这一生态系统中具有极高的黏菌物种多样性(Novozhilov et al. 2006;Novozhilov & Schnittler 2008;Estrada-Torres et al. 2009;Lado et al. 2011;2013;2014;Schnittler et al. 2013;Wrigley de Basanta et al. 2013).另外,一些特殊生境中分布的黏菌也引起广泛关注,如发生于春季融雪带的雪线黏菌陆续报道于西班牙格雷多山(Lado et al. 2005)、比利牛斯山(Lado & Ronikier 2008,2009)、波兰喀尔巴阡山(Ronikier et al. 2008)、澳大利亚科西阿斯科山(Stephenson & Shadwick 2009)和俄罗斯高加索山(Novozhilov et al. 2012)等山脉.在极地生态系统开展的黏菌调查研究表明,阿拉斯加、冰岛、格陵兰岛和俄罗斯等北极地区广泛分布着北温带的稀有种(Stephenson et al. 2000);类似地,亚南极地区麦夸里岛也发现了一些罕见种(Wrigley de Basanta et al. 2010),表明极地生态系统拥有较高的黏菌物种特有性. ...
Barcoding myxomycetes with molecular markers- challenges and opportunities
1
2017
... 扩增子高通量测序技术为黏菌生态学研究提供了极大的便利,在实际研究中选择合适的条形码基因及其特异性引物尤为重要.目前,SSU rRNA基因V2区已被证明是黏菌理想的条形码基因(Schnittler et al. 2017),但受限于黏菌内部两个演化支系(亮孢黏菌分支Lucidosporidia和囊轴黏菌分支Columellidia)间的遗传分歧,难以设计用于扩增子测序分析的通用引物.近期,Wang et al.(2021)筛选出黏菌SSU rRNA基因的通用引物组合,有望实现对环境中黏菌群落信息的完整刻画.另一方面,已报道的扩增子测序研究中存在大量未能注释的序列,为后续的数据解读带来了困难,未来应在广泛采集和准确鉴定物种的基础上,进一步扩充参考基因序列的种类和数量,同时,考虑到黏菌遗传多样性显著的地域性特点,建立我国本土的标记基因数据库也尤为必要. ...
Ecology of sandstone ravine myxomycetes from Saxonian Switzerland (Germany)
2
2010
... 生态位是指物种利用各种资源环境的总和,是研究物种间相互作用、对环境的适应性以及解释物种间竞争和共存机制的重要理论(Leibold 1995).在对黏菌种群生态位的研究中,通常选择几个重要因子进行生态位分析,如海拔、气温、降水量和基质类型等(Stephenson & Rojas 2017).总体来看,黏菌生态位宽度与物种的丰富度并无显著相关性,多数黏菌种类的生态位宽度较大,也即对环境的适应能力较强,是“生态泛化者”,而少数黏菌种类趋向成为特化种(Stephenson 1988).不同生态系统类型或基质栖生黏菌生态位也各具特点,如森林生态系统中的黏菌生态位宽度较大(Stephenson 1988;Rojas & Stephenson 2007),砂岩隐花植物生态系统中的黏菌生态位宽度较小(Schnittler et al. 2010);腐木栖生黏菌生态位宽度大,凋落物栖生的黏菌生态宽度小(Schnittler 2001;Rojas et al. 2010). ...
... 生态位重叠分析表明,黏菌物种间普遍具有较高的生态位重叠值(Stephenson 1988;Rojas & Stephenson 2007;Schnittler et al. 2010),即物种对环境资源有着相似的需求.但相对而言,子实体小型且原生质团隐型或原始型的树皮栖生黏菌生态位重叠度高,种间联结度高,而显型原生质团的凋落物栖生黏菌重叠度低,存在明显的种间竞争(Schnittler 2001).此外,黏菌的属内种间物种的生态位重叠度与属间物种生态位重叠度并无显著差异,反映了亲缘关系较远的黏菌演化出类似的生态策略(Rojas & Stephenson 2007). ...
Community of dark-spored myxomycetes in ground litter and soil of taiga forest (Nizhne-Svirskiy Reserve, Russia) revealed by DNA metabarcoding
2
2019
... 高通量测序技术的应用极大地增加了扩增子测序的测序深度,目前从草原(Fiore- Donno et al. 2016)、融雪带(Borg Dahl et al. 2018b)和森林(Borg Dahl et al. 2019;Gao et al. 2019;Shchepin et al. 2019)土壤中所报道的暗孢黏菌OTUs数量在200-300之间.在群落组成方面,已有18属暗孢黏菌报道分布于土壤中,其中亮皮菌属Lamproderma、发网菌属Stemonitis、裂皮菌属Meriderma和双皮菌属Diderma为优势属,一些典型的林中凋落物栖生黏菌如高杯菌Craterium minutum、光果菌Leocarpus fragilis等也被发现分布于土壤中(Gao et al. 2019;Shchepin et al. 2019).土壤中黏菌的分布格局也得到进一步认识,例如,森林火灾后不同年份土壤的黏菌组成显著不同,表明黏菌群落存在显著的演替规律;不同植被土壤的黏菌群落存在显著差异,并主要由土壤pH驱动(Borg Dahl et al. 2019;Gao et al. 2019).我们整合了目前已发表的土壤暗孢黏菌高通量测序数据(PRJNA418896;PRJNA491744;PRJNA565474),通过非度量多维尺度(non-metric multidimensional scaling,NMDS)分析发现,德国阿尔卑斯山地森林、俄罗斯西北部泰加林和中国河南宝天曼保护区温带森林的土壤黏菌群落组成显著不同(图2),这也为黏菌的大尺度地带性分布格局提供了佐证. ...
... ;Shchepin et al. 2019).土壤中黏菌的分布格局也得到进一步认识,例如,森林火灾后不同年份土壤的黏菌组成显著不同,表明黏菌群落存在显著的演替规律;不同植被土壤的黏菌群落存在显著差异,并主要由土壤pH驱动(Borg Dahl et al. 2019;Gao et al. 2019).我们整合了目前已发表的土壤暗孢黏菌高通量测序数据(PRJNA418896;PRJNA491744;PRJNA565474),通过非度量多维尺度(non-metric multidimensional scaling,NMDS)分析发现,德国阿尔卑斯山地森林、俄罗斯西北部泰加林和中国河南宝天曼保护区温带森林的土壤黏菌群落组成显著不同(图2),这也为黏菌的大尺度地带性分布格局提供了佐证. ...
Species diversity of myxomycetes in Huanglian Mountain National Nature Reserve of Yunnan Province, China
0
2014
Distribution and ecology of myxomycetes in temperate forests. I. Patterns of occurrence in the upland forests of southwestern Virginia
5
1988
... 生态位是指物种利用各种资源环境的总和,是研究物种间相互作用、对环境的适应性以及解释物种间竞争和共存机制的重要理论(Leibold 1995).在对黏菌种群生态位的研究中,通常选择几个重要因子进行生态位分析,如海拔、气温、降水量和基质类型等(Stephenson & Rojas 2017).总体来看,黏菌生态位宽度与物种的丰富度并无显著相关性,多数黏菌种类的生态位宽度较大,也即对环境的适应能力较强,是“生态泛化者”,而少数黏菌种类趋向成为特化种(Stephenson 1988).不同生态系统类型或基质栖生黏菌生态位也各具特点,如森林生态系统中的黏菌生态位宽度较大(Stephenson 1988;Rojas & Stephenson 2007),砂岩隐花植物生态系统中的黏菌生态位宽度较小(Schnittler et al. 2010);腐木栖生黏菌生态位宽度大,凋落物栖生的黏菌生态宽度小(Schnittler 2001;Rojas et al. 2010). ...
... ).不同生态系统类型或基质栖生黏菌生态位也各具特点,如森林生态系统中的黏菌生态位宽度较大(Stephenson 1988;Rojas & Stephenson 2007),砂岩隐花植物生态系统中的黏菌生态位宽度较小(Schnittler et al. 2010);腐木栖生黏菌生态位宽度大,凋落物栖生的黏菌生态宽度小(Schnittler 2001;Rojas et al. 2010). ...
... 生态位重叠分析表明,黏菌物种间普遍具有较高的生态位重叠值(Stephenson 1988;Rojas & Stephenson 2007;Schnittler et al. 2010),即物种对环境资源有着相似的需求.但相对而言,子实体小型且原生质团隐型或原始型的树皮栖生黏菌生态位重叠度高,种间联结度高,而显型原生质团的凋落物栖生黏菌重叠度低,存在明显的种间竞争(Schnittler 2001).此外,黏菌的属内种间物种的生态位重叠度与属间物种生态位重叠度并无显著差异,反映了亲缘关系较远的黏菌演化出类似的生态策略(Rojas & Stephenson 2007). ...
... 黏菌的纬度分布格局研究表明,黏菌的物种丰富度与纬度呈正相关关系,林型、土壤理化性质和基质pH是这一分布格局的主要驱动因子(Rojas et al. 2011).同时,在温带与热带森林黏菌多样性的比较研究中,温带森林均高于热带森林,热带地区过高的降水量和林下快速降解的凋落物不利于黏菌子实体的形成,同时,封闭的森林冠层使气流减少也可能会阻碍黏菌孢子的扩散(Stephenson et al. 1993;Cruz et al. 2014). ...
... 黏菌群落的时间动态一般由不同季节的湿热条件决定(Stephenson 1988;Takahashi & Hada 2012).基于野外采集和湿室培养研究热带地区的低海拔森林和农田生态系统发现,雨季黏菌的丰富度和多样性显著高于旱季(Tran et al. 2006;2008;Ko Ko et al. 2011).宋天鹏和陈双林(2014)在中国云南黄连山保护区较高海拔的亚热带森林的研究显示,雨季的黏菌多样性低于旱季,该研究通过湿室培养法收集黏菌子实体,反映出旱季的基物上存在更多黏菌休眠体.一些黏菌的发生具有季节性,造就了黏菌群落组成的季节性差异,如发网菌目黏菌多发生于雨季、绒泡菌目多发生于旱季(Ko Ko et al. 2011).日本温带森林凋落物栖生黏菌具有明显的季节发生规律,根据物种组成分为早期(4-5月)、中期(6-8月)和晚期(10-11月)3个阶段(Takahashi & Hada 2012).类似地,中国河南宝天曼保护区林中凋落物栖生黏菌群落在春、夏、秋3季也显著不同,基物类型及月平均气温与降水等气象因子是影响黏菌群落组成的主要因素(Gao et al. 2018). ...
A comparative biogeographical study of myxomycetes in the mid-Appalachians of eastern North America and two regions of India
0
1993
Myxomycetes of subantarctic Macquarie Island
2
2007
... 原生动物的生物地理分布模式,存在两种假说:一为原生动物在全球范围内呈随机分布,其分布主要由所处生境中的环境因子决定,即“everything is everywhere, but the environment selects”(Finlay 2002);二为与之相对应的地带性分布(moderate endemicity),指物种的分布受扩散限制的影响(Foissner 2006).黏菌归属于原生动物界已经获得普遍接受(Alexopoulos et al. 1996),其孢子能够借助气流进行长距离散播(Kamono et al. 2009a),具有较强的扩散能力.基于标本采集的物种调查和形态分类学研究结果一方面为黏菌的随机分布提供了证据支持,如许多黏菌物种呈现出跨大陆的分布格局(Stephenson et al. 2008).黏菌物种调查研究同时显示,偏远海岛的物种组成与大陆类似而无特有种存在,包括美国夏威夷群岛(Eliasson 1991)和澳大利亚麦夸里岛(Stephenson et al. 2007)等.另一方面,一些研究结果表明黏菌也可能受扩散限制的影响,而呈现地带性分布,如裂瓣菌Barbeyella minutissima集中分布于温带森林,而圆孢鹅绒菌Ceratiomyxa sphaerosperma仅知分布于美洲的热带地区(Stephenson & Rojas 2017).Dagamac et al.(2017b)研究了新热带和古热带地区两种森林生态系统的黏菌群落组成,发现一些物种的分布局限于特定的地理区域,地理距离而非生境差异对黏菌群落组成影响更大. ...
... 黏菌群落的垂直分布格局研究显示,黏菌的物种多样性与海拔总体呈负相关关系,但驱动这一分布格局的环境因子各不相同,例如,Stephenson et al.(2004)认为在厄瓜多尔热带云雾林中过高的湿度条件是高海拔样地黏菌多样性低的主要原因;Rojas & Stephenson(2007)研究发现pH是驱动可可岛黏菌多样性黏菌垂直分布的关键因素;魏滨等(2016)认为不同海拔梯度下的气流差异驱动了中国广东鼎湖山自然保护区黏菌垂直分布的形成;Rojas et al.(2016)选择植被类型均一的海拔梯度样带进行研究后发现,黏菌多样性和群落组成均与海拔不相关,认为黏菌的垂直分布格局应归因于不同海拔的植被差异. ...
Distribution and ecology of myxomycetes in high‐latitude regions of the Northern Hemisphere
1
2000
... 2003年,随着“全球黏菌生物多样性”(PBI: Global Biodiversity of Eumycetozoans)项目的实施,黏菌分类和多样性研究也拓展至中亚和南美沙漠.出乎意料的是,相关研究借助湿室培养技术,揭示出这一生态系统中具有极高的黏菌物种多样性(Novozhilov et al. 2006;Novozhilov & Schnittler 2008;Estrada-Torres et al. 2009;Lado et al. 2011;2013;2014;Schnittler et al. 2013;Wrigley de Basanta et al. 2013).另外,一些特殊生境中分布的黏菌也引起广泛关注,如发生于春季融雪带的雪线黏菌陆续报道于西班牙格雷多山(Lado et al. 2005)、比利牛斯山(Lado & Ronikier 2008,2009)、波兰喀尔巴阡山(Ronikier et al. 2008)、澳大利亚科西阿斯科山(Stephenson & Shadwick 2009)和俄罗斯高加索山(Novozhilov et al. 2012)等山脉.在极地生态系统开展的黏菌调查研究表明,阿拉斯加、冰岛、格陵兰岛和俄罗斯等北极地区广泛分布着北温带的稀有种(Stephenson et al. 2000);类似地,亚南极地区麦夸里岛也发现了一些罕见种(Wrigley de Basanta et al. 2010),表明极地生态系统拥有较高的黏菌物种特有性. ...
Myxomycetes. Biology, Systematics, Biogeography and Ecology
3
2017
... 原生动物的生物地理分布模式,存在两种假说:一为原生动物在全球范围内呈随机分布,其分布主要由所处生境中的环境因子决定,即“everything is everywhere, but the environment selects”(Finlay 2002);二为与之相对应的地带性分布(moderate endemicity),指物种的分布受扩散限制的影响(Foissner 2006).黏菌归属于原生动物界已经获得普遍接受(Alexopoulos et al. 1996),其孢子能够借助气流进行长距离散播(Kamono et al. 2009a),具有较强的扩散能力.基于标本采集的物种调查和形态分类学研究结果一方面为黏菌的随机分布提供了证据支持,如许多黏菌物种呈现出跨大陆的分布格局(Stephenson et al. 2008).黏菌物种调查研究同时显示,偏远海岛的物种组成与大陆类似而无特有种存在,包括美国夏威夷群岛(Eliasson 1991)和澳大利亚麦夸里岛(Stephenson et al. 2007)等.另一方面,一些研究结果表明黏菌也可能受扩散限制的影响,而呈现地带性分布,如裂瓣菌Barbeyella minutissima集中分布于温带森林,而圆孢鹅绒菌Ceratiomyxa sphaerosperma仅知分布于美洲的热带地区(Stephenson & Rojas 2017).Dagamac et al.(2017b)研究了新热带和古热带地区两种森林生态系统的黏菌群落组成,发现一些物种的分布局限于特定的地理区域,地理距离而非生境差异对黏菌群落组成影响更大. ...
... 早期的黏菌生物学围绕黏菌生活史开展了大量研究,其中一些研究揭示了孢子萌发、黏变形体生长、原生质团行为等与所处的生态环境,包括温度、湿度、pH等一系列环境因素之间的关系(Madelin 1984),可以将这些研究归纳到个体生态学的范畴.Maimoni- Rodella & Gottsberger(1980)定量分析了巴西热带森林中的黏菌群落与基质类型、温度和降水量的关系,开启了黏菌的群落生态学研究.目前,绝大多数黏菌生态学研究首先通过子实体调查和分类鉴定,获得一定区域或生境内的黏菌物种组成与丰度信息;再应用数量生态学的分析手段,揭示黏菌群落的物种分布格局和影响因素等(Stephenson & Rojas 2017).这种基于子实体调查的方法不可避免地忽略了环境中一些无法发育为子实体的黏菌营养体(李玉等 2021),因此,许多研究者尝试将DNA分子标记和指纹图谱技术用于解析环境中的黏菌群落,如PCR-DGGE(Kamono & Fukui 2006;Kamono et al. 2009b)、PCR-T-RFLP(Hoppe & Schnittler 2015)等,标志着黏菌生态学研究已进入分子时代.当前,黏菌DNA条形码与高通量测序结合产生的扩增子高通量测序为黏菌生态学研究提供了新方法,通过环境基因组DNA提取、条形码基因PCR扩增、产物高通量测序和序列分类注释,已实现了不依赖子实体调查的群落定性、定量分析(Borg Dahl et al. 2018a). ...
... 生态位是指物种利用各种资源环境的总和,是研究物种间相互作用、对环境的适应性以及解释物种间竞争和共存机制的重要理论(Leibold 1995).在对黏菌种群生态位的研究中,通常选择几个重要因子进行生态位分析,如海拔、气温、降水量和基质类型等(Stephenson & Rojas 2017).总体来看,黏菌生态位宽度与物种的丰富度并无显著相关性,多数黏菌种类的生态位宽度较大,也即对环境的适应能力较强,是“生态泛化者”,而少数黏菌种类趋向成为特化种(Stephenson 1988).不同生态系统类型或基质栖生黏菌生态位也各具特点,如森林生态系统中的黏菌生态位宽度较大(Stephenson 1988;Rojas & Stephenson 2007),砂岩隐花植物生态系统中的黏菌生态位宽度较小(Schnittler et al. 2010);腐木栖生黏菌生态位宽度大,凋落物栖生的黏菌生态宽度小(Schnittler 2001;Rojas et al. 2010). ...
Ecological characterization of a tropical myxomycete assemblage—Maquipucuna Cloud Forest Reserve, Ecuador
1
2004
... 黏菌群落的垂直分布格局研究显示,黏菌的物种多样性与海拔总体呈负相关关系,但驱动这一分布格局的环境因子各不相同,例如,Stephenson et al.(2004)认为在厄瓜多尔热带云雾林中过高的湿度条件是高海拔样地黏菌多样性低的主要原因;Rojas & Stephenson(2007)研究发现pH是驱动可可岛黏菌多样性黏菌垂直分布的关键因素;魏滨等(2016)认为不同海拔梯度下的气流差异驱动了中国广东鼎湖山自然保护区黏菌垂直分布的形成;Rojas et al.(2016)选择植被类型均一的海拔梯度样带进行研究后发现,黏菌多样性和群落组成均与海拔不相关,认为黏菌的垂直分布格局应归因于不同海拔的植被差异. ...
Myxomycetes of the Great Smoky Mountains National Park
1
2001
... 自Panckow在1654年首次记录并描述粉瘤菌Lycogala epidendrum开始,尤其是Rostafinski在1873年建立第一个黏菌现代分类系统以来(Alexopoulos et al. 1996),黏菌工作者陆续在世界各地开展了大量的标本采集和经典分类的研究工作,出版了众多专著.但在很长一段时期内,研究区域主要集中于北半球的温带地区,其中以北美、欧洲和亚洲的温带森林生态系统最为突出(Stephenson et al. 2008),不仅有众多的论文,而且有许多地区志,包括北美的《The North American slime-moulds》和《The Mycetozoa of North America》,欧洲的《The Myxomycetes of Great Britain》《A monograph of the Mycetozoa— Being a descriptive catalogue of the species in the herbarium of the British Museum》《Mycological flora of European and Asian Russia. The slime molds》《A guide to temperate Myxomycetes》《Die Myxomyceten Deutschlands und des angrenzenden Alpenraumes unter besonderer Berücksichtigung Österreichs》《The Myxomycetes of Britain and Ireland. An identification handbook》和《Les Myxomycètes》,亚洲的《The Myxomycetes of Japan》《The Myxomycetes of India》《Taxonomy of the Indian Myxomycetes》《The myxomycete biota of Japan》和《中国真菌志·黏菌卷》等等.从已有调查结果来看,温带落叶阔叶林可能是地球上黏菌物种多样性最高的生态系统,如美国大雾山国家公园报道黏菌168种(Stephenson et al. 2001);俄罗斯锡霍特-阿林保护区报道黏菌158种(Novozhilov et al. 2017b);中国小兴安岭地区报道黏菌152种(赵凤云等 2019). ...
Myxomycete diversity and distribution from the fossil record to the present
2
2008
... 自Panckow在1654年首次记录并描述粉瘤菌Lycogala epidendrum开始,尤其是Rostafinski在1873年建立第一个黏菌现代分类系统以来(Alexopoulos et al. 1996),黏菌工作者陆续在世界各地开展了大量的标本采集和经典分类的研究工作,出版了众多专著.但在很长一段时期内,研究区域主要集中于北半球的温带地区,其中以北美、欧洲和亚洲的温带森林生态系统最为突出(Stephenson et al. 2008),不仅有众多的论文,而且有许多地区志,包括北美的《The North American slime-moulds》和《The Mycetozoa of North America》,欧洲的《The Myxomycetes of Great Britain》《A monograph of the Mycetozoa— Being a descriptive catalogue of the species in the herbarium of the British Museum》《Mycological flora of European and Asian Russia. The slime molds》《A guide to temperate Myxomycetes》《Die Myxomyceten Deutschlands und des angrenzenden Alpenraumes unter besonderer Berücksichtigung Österreichs》《The Myxomycetes of Britain and Ireland. An identification handbook》和《Les Myxomycètes》,亚洲的《The Myxomycetes of Japan》《The Myxomycetes of India》《Taxonomy of the Indian Myxomycetes》《The myxomycete biota of Japan》和《中国真菌志·黏菌卷》等等.从已有调查结果来看,温带落叶阔叶林可能是地球上黏菌物种多样性最高的生态系统,如美国大雾山国家公园报道黏菌168种(Stephenson et al. 2001);俄罗斯锡霍特-阿林保护区报道黏菌158种(Novozhilov et al. 2017b);中国小兴安岭地区报道黏菌152种(赵凤云等 2019). ...
... 原生动物的生物地理分布模式,存在两种假说:一为原生动物在全球范围内呈随机分布,其分布主要由所处生境中的环境因子决定,即“everything is everywhere, but the environment selects”(Finlay 2002);二为与之相对应的地带性分布(moderate endemicity),指物种的分布受扩散限制的影响(Foissner 2006).黏菌归属于原生动物界已经获得普遍接受(Alexopoulos et al. 1996),其孢子能够借助气流进行长距离散播(Kamono et al. 2009a),具有较强的扩散能力.基于标本采集的物种调查和形态分类学研究结果一方面为黏菌的随机分布提供了证据支持,如许多黏菌物种呈现出跨大陆的分布格局(Stephenson et al. 2008).黏菌物种调查研究同时显示,偏远海岛的物种组成与大陆类似而无特有种存在,包括美国夏威夷群岛(Eliasson 1991)和澳大利亚麦夸里岛(Stephenson et al. 2007)等.另一方面,一些研究结果表明黏菌也可能受扩散限制的影响,而呈现地带性分布,如裂瓣菌Barbeyella minutissima集中分布于温带森林,而圆孢鹅绒菌Ceratiomyxa sphaerosperma仅知分布于美洲的热带地区(Stephenson & Rojas 2017).Dagamac et al.(2017b)研究了新热带和古热带地区两种森林生态系统的黏菌群落组成,发现一些物种的分布局限于特定的地理区域,地理距离而非生境差异对黏菌群落组成影响更大. ...
Nivicolous myxomycetes from alpine areas of south-eastern Australia
1
2009
... 2003年,随着“全球黏菌生物多样性”(PBI: Global Biodiversity of Eumycetozoans)项目的实施,黏菌分类和多样性研究也拓展至中亚和南美沙漠.出乎意料的是,相关研究借助湿室培养技术,揭示出这一生态系统中具有极高的黏菌物种多样性(Novozhilov et al. 2006;Novozhilov & Schnittler 2008;Estrada-Torres et al. 2009;Lado et al. 2011;2013;2014;Schnittler et al. 2013;Wrigley de Basanta et al. 2013).另外,一些特殊生境中分布的黏菌也引起广泛关注,如发生于春季融雪带的雪线黏菌陆续报道于西班牙格雷多山(Lado et al. 2005)、比利牛斯山(Lado & Ronikier 2008,2009)、波兰喀尔巴阡山(Ronikier et al. 2008)、澳大利亚科西阿斯科山(Stephenson & Shadwick 2009)和俄罗斯高加索山(Novozhilov et al. 2012)等山脉.在极地生态系统开展的黏菌调查研究表明,阿拉斯加、冰岛、格陵兰岛和俄罗斯等北极地区广泛分布着北温带的稀有种(Stephenson et al. 2000);类似地,亚南极地区麦夸里岛也发现了一些罕见种(Wrigley de Basanta et al. 2010),表明极地生态系统拥有较高的黏菌物种特有性. ...
Seasonal occurrence and distribution of myxomycetes on different types of leaf litter in a warm temperate forest of western Japan
2
2012
... 黏菌群落的时间动态一般由不同季节的湿热条件决定(Stephenson 1988;Takahashi & Hada 2012).基于野外采集和湿室培养研究热带地区的低海拔森林和农田生态系统发现,雨季黏菌的丰富度和多样性显著高于旱季(Tran et al. 2006;2008;Ko Ko et al. 2011).宋天鹏和陈双林(2014)在中国云南黄连山保护区较高海拔的亚热带森林的研究显示,雨季的黏菌多样性低于旱季,该研究通过湿室培养法收集黏菌子实体,反映出旱季的基物上存在更多黏菌休眠体.一些黏菌的发生具有季节性,造就了黏菌群落组成的季节性差异,如发网菌目黏菌多发生于雨季、绒泡菌目多发生于旱季(Ko Ko et al. 2011).日本温带森林凋落物栖生黏菌具有明显的季节发生规律,根据物种组成分为早期(4-5月)、中期(6-8月)和晚期(10-11月)3个阶段(Takahashi & Hada 2012).类似地,中国河南宝天曼保护区林中凋落物栖生黏菌群落在春、夏、秋3季也显著不同,基物类型及月平均气温与降水等气象因子是影响黏菌群落组成的主要因素(Gao et al. 2018). ...
... ).日本温带森林凋落物栖生黏菌具有明显的季节发生规律,根据物种组成分为早期(4-5月)、中期(6-8月)和晚期(10-11月)3个阶段(Takahashi & Hada 2012).类似地,中国河南宝天曼保护区林中凋落物栖生黏菌群落在春、夏、秋3季也显著不同,基物类型及月平均气温与降水等气象因子是影响黏菌群落组成的主要因素(Gao et al. 2018). ...
Distribution and occurrence of myxomycetes in tropical forests of northern Thailand
2
2006
... 热带、亚热带黏菌的早期研究仅限于零星报道.20世纪70年代,Farr(1976)系统整理了前人文献资料并记述了新热带区280种黏菌.在此基础上,Lado & Wrigley de Basanta(2008)重新梳理了分布于新热带地区的黏菌物种,共计431种.近年,研究人员陆续在我国热带和亚热带森林(闫淑珍等2012;Liu et al. 2013;魏滨等 2016;Gao et al. 2018;Li et al. 2021)、东南亚热带雨林开展了系统调查(Tran et al. 2006;2008;Ko Ko et al. 2010,2012,2013;Dagamac et al. 2011,2014,2017a;Novozhilov et al. 2017a,2020),极大地丰富了黏菌物种的地理分布和区系特征. ...
... 黏菌群落的时间动态一般由不同季节的湿热条件决定(Stephenson 1988;Takahashi & Hada 2012).基于野外采集和湿室培养研究热带地区的低海拔森林和农田生态系统发现,雨季黏菌的丰富度和多样性显著高于旱季(Tran et al. 2006;2008;Ko Ko et al. 2011).宋天鹏和陈双林(2014)在中国云南黄连山保护区较高海拔的亚热带森林的研究显示,雨季的黏菌多样性低于旱季,该研究通过湿室培养法收集黏菌子实体,反映出旱季的基物上存在更多黏菌休眠体.一些黏菌的发生具有季节性,造就了黏菌群落组成的季节性差异,如发网菌目黏菌多发生于雨季、绒泡菌目多发生于旱季(Ko Ko et al. 2011).日本温带森林凋落物栖生黏菌具有明显的季节发生规律,根据物种组成分为早期(4-5月)、中期(6-8月)和晚期(10-11月)3个阶段(Takahashi & Hada 2012).类似地,中国河南宝天曼保护区林中凋落物栖生黏菌群落在春、夏、秋3季也显著不同,基物类型及月平均气温与降水等气象因子是影响黏菌群落组成的主要因素(Gao et al. 2018). ...
Distribution and occurrence of myxomycetes on agricultural ground litter and forest floor litter in Thailand
2
2008
... 热带、亚热带黏菌的早期研究仅限于零星报道.20世纪70年代,Farr(1976)系统整理了前人文献资料并记述了新热带区280种黏菌.在此基础上,Lado & Wrigley de Basanta(2008)重新梳理了分布于新热带地区的黏菌物种,共计431种.近年,研究人员陆续在我国热带和亚热带森林(闫淑珍等2012;Liu et al. 2013;魏滨等 2016;Gao et al. 2018;Li et al. 2021)、东南亚热带雨林开展了系统调查(Tran et al. 2006;2008;Ko Ko et al. 2010,2012,2013;Dagamac et al. 2011,2014,2017a;Novozhilov et al. 2017a,2020),极大地丰富了黏菌物种的地理分布和区系特征. ...
... 黏菌群落的时间动态一般由不同季节的湿热条件决定(Stephenson 1988;Takahashi & Hada 2012).基于野外采集和湿室培养研究热带地区的低海拔森林和农田生态系统发现,雨季黏菌的丰富度和多样性显著高于旱季(Tran et al. 2006;2008;Ko Ko et al. 2011).宋天鹏和陈双林(2014)在中国云南黄连山保护区较高海拔的亚热带森林的研究显示,雨季的黏菌多样性低于旱季,该研究通过湿室培养法收集黏菌子实体,反映出旱季的基物上存在更多黏菌休眠体.一些黏菌的发生具有季节性,造就了黏菌群落组成的季节性差异,如发网菌目黏菌多发生于雨季、绒泡菌目多发生于旱季(Ko Ko et al. 2011).日本温带森林凋落物栖生黏菌具有明显的季节发生规律,根据物种组成分为早期(4-5月)、中期(6-8月)和晚期(10-11月)3个阶段(Takahashi & Hada 2012).类似地,中国河南宝天曼保护区林中凋落物栖生黏菌群落在春、夏、秋3季也显著不同,基物类型及月平均气温与降水等气象因子是影响黏菌群落组成的主要因素(Gao et al. 2018). ...
Design of potentially universal SSU primers in myxomycetes using next-generation sequencing
1
2021
... 扩增子高通量测序技术为黏菌生态学研究提供了极大的便利,在实际研究中选择合适的条形码基因及其特异性引物尤为重要.目前,SSU rRNA基因V2区已被证明是黏菌理想的条形码基因(Schnittler et al. 2017),但受限于黏菌内部两个演化支系(亮孢黏菌分支Lucidosporidia和囊轴黏菌分支Columellidia)间的遗传分歧,难以设计用于扩增子测序分析的通用引物.近期,Wang et al.(2021)筛选出黏菌SSU rRNA基因的通用引物组合,有望实现对环境中黏菌群落信息的完整刻画.另一方面,已报道的扩增子测序研究中存在大量未能注释的序列,为后续的数据解读带来了困难,未来应在广泛采集和准确鉴定物种的基础上,进一步扩充参考基因序列的种类和数量,同时,考虑到黏菌遗传多样性显著的地域性特点,建立我国本土的标记基因数据库也尤为必要. ...
Species composition and diversity of myxomycetes in Dinghu Mountain Nature Reserve
0
2016
Global distribution and molecular diversity of Didymium difforme
1
2011
... 黏菌形态种下可能存在多个隐存种,这些隐存种之间彼此存在生殖隔离,但难以从形态上区分(Feng & Schnittler 2015;Feng et al. 2016),进而干扰所研究物种的地理分布模式.目前,在许多黏菌的生物地理分布研究中增加了分子标记手段,通过基因型的差异更准确地反映物种分布,但结论也不尽相同.Winsett & Stephenson(2011)以mt SSU rRNA基因分析了畸形钙皮菌Didymium difforme的地理分布规律,13种mt SSU rRNA基因型为随机分布.Hoppe(2013)研究显示煤绒菌黄色变种Fuligo septica var. flava可根据mt SSU rRNA基因分为3个分支,分支间的遗传距离与地理距离不相关.Aguilar et al.(2014)采用SSU rRNA基因系统发育分析和环境生态位模拟,分析了美洲的暗孢钙丝菌Badhamia melanospora种群遗传分化,结果显示,南北美洲的暗孢钙丝菌B. melanospora按照大陆的分离完全隔断为南北两个基因型的种群,大部分种群局限分布在一个较为固定的地理范围内.Dagamac et al.(2017c)通过SSU rRNA基因序列分析发现蛇形半网菌Hemitrichia serpula包含3个主要的系统发育群,不同种群的地理分布、形态特征和EF-1α基因序列也显著不同.Janik et al.(2020)研究了雪生钙皮菌Didymium nivicola的SSU rRNA和EF-1α基因多态性,发现在安第斯山脉的种内遗传多样性最高,包含17种SSU核糖体型和12种EF-1α基因型,而北半球种群的遗传背景则比较一致,因此认为安第斯山脉可能是雪生钙皮菌D. nivicola的遗传多样性分化中心. ...
Biodiversity of myxomycetes in subantarctic forests of Patagonia and Tierra del Fuego, Argentina
1
2010
... 2003年,随着“全球黏菌生物多样性”(PBI: Global Biodiversity of Eumycetozoans)项目的实施,黏菌分类和多样性研究也拓展至中亚和南美沙漠.出乎意料的是,相关研究借助湿室培养技术,揭示出这一生态系统中具有极高的黏菌物种多样性(Novozhilov et al. 2006;Novozhilov & Schnittler 2008;Estrada-Torres et al. 2009;Lado et al. 2011;2013;2014;Schnittler et al. 2013;Wrigley de Basanta et al. 2013).另外,一些特殊生境中分布的黏菌也引起广泛关注,如发生于春季融雪带的雪线黏菌陆续报道于西班牙格雷多山(Lado et al. 2005)、比利牛斯山(Lado & Ronikier 2008,2009)、波兰喀尔巴阡山(Ronikier et al. 2008)、澳大利亚科西阿斯科山(Stephenson & Shadwick 2009)和俄罗斯高加索山(Novozhilov et al. 2012)等山脉.在极地生态系统开展的黏菌调查研究表明,阿拉斯加、冰岛、格陵兰岛和俄罗斯等北极地区广泛分布着北温带的稀有种(Stephenson et al. 2000);类似地,亚南极地区麦夸里岛也发现了一些罕见种(Wrigley de Basanta et al. 2010),表明极地生态系统拥有较高的黏菌物种特有性. ...
Biodiversity studies of myxomycetes in Madagascar
1
2013
... 2003年,随着“全球黏菌生物多样性”(PBI: Global Biodiversity of Eumycetozoans)项目的实施,黏菌分类和多样性研究也拓展至中亚和南美沙漠.出乎意料的是,相关研究借助湿室培养技术,揭示出这一生态系统中具有极高的黏菌物种多样性(Novozhilov et al. 2006;Novozhilov & Schnittler 2008;Estrada-Torres et al. 2009;Lado et al. 2011;2013;2014;Schnittler et al. 2013;Wrigley de Basanta et al. 2013).另外,一些特殊生境中分布的黏菌也引起广泛关注,如发生于春季融雪带的雪线黏菌陆续报道于西班牙格雷多山(Lado et al. 2005)、比利牛斯山(Lado & Ronikier 2008,2009)、波兰喀尔巴阡山(Ronikier et al. 2008)、澳大利亚科西阿斯科山(Stephenson & Shadwick 2009)和俄罗斯高加索山(Novozhilov et al. 2012)等山脉.在极地生态系统开展的黏菌调查研究表明,阿拉斯加、冰岛、格陵兰岛和俄罗斯等北极地区广泛分布着北温带的稀有种(Stephenson et al. 2000);类似地,亚南极地区麦夸里岛也发现了一些罕见种(Wrigley de Basanta et al. 2010),表明极地生态系统拥有较高的黏菌物种特有性. ...
Known species of Myxomycetes from tropical China
0
2012
Species diversity of myxomycetes in two forests of the Lesser Khinggan Mountains, China
0
2019
黏菌物种多样性及其与影响因子关系的研究进展
1
2021
... 黏菌最常见于林下潮湿的凋落物、树皮、倒木、食草动物粪便和土壤甚至活的动物体中,能以伪足取食基质最细微孔隙中的细菌、真菌及其他微生物(Liu et al. 2015;李敏和陈双林 2021).黏菌的这一摄食活动可增加植物凋落物分解过程中的CO2释放速率和质量损失(Geisen et al. 2021),促进N、P、K等元素的循环(Fukasawa et al. 2017),是森林生态系统物质循环和能量流动过程中不可替代的参与者.黏菌的地理分布和生态学研究是当前黏菌生物学研究的重要领域之一,已有研究不同程度地揭示了黏菌的地理分布特征、生态位特征、时空分布格局以及与其他生物或非生物因子的关系等,对于理解黏菌的生态系统服务和功能具有重要作用.本文对黏菌分布格局和生态学研究中的进展性成果进行综述,并结合当前研究动态,对今后研究提出展望. ...
中国真菌志. 黏菌卷I,II
3
2008
... 黏菌Myxogastrea是原生动物界Protozoa变形虫门Amoebozoa中物种数量最为庞大的一个单系类群(Leontyev et al. 2019).生活史兼具原生动物和真菌的共同特征,营养阶段为单核的黏变形体(或游动胞)和多核的原生质团,繁殖阶段形成内含单倍体孢子的子实体(李玉等 2008).黏菌分类鉴定主要依靠子实体的形态特征,如基质层有无、柄的形态与质地、囊轴形态、孢丝有无与形态、孢子大小与纹饰等(李玉等 2008;Leontyev et al. 2019).自17世纪中叶起,菌物学工作者陆续在世界范围内开展了一系列黏菌资源的调查工作,迄今全球已被描述并认可的黏菌约1 000种(Lado 2005-2021).传统的黏菌分类系统将黏菌纲分为3亚纲6目(李玉等 2008),即鹅绒菌亚纲Ceratiomyxomycetidae鹅绒菌目Ceratiomyxales,腹黏菌亚纲Myxogastromycetidae刺轴菌目Echinosteliales、无丝菌目Liceales、团毛菌目Trichiales和绒泡菌目Physarales,发网菌亚纲Stemonitomycetidae发网菌目Stemonitales.晚近,Leontyev et al.(2019)结合分子系统学和形态分类学研究对黏菌分类系统做出较大调整,在保留主要的固有分类单元并遵循系统发育单系性的原则下,在黏菌纲中排除鹅绒菌目Ceratiomyxales,并且基于浅色孢子分支成立浅孢黏菌亚纲Lucisporomycetidae,其下含有无丝菌目Liceales、团毛菌目Trichiales、筛菌目Cribrariales和孔膜菌目Reticulariales;以子实体具有囊轴成立囊轴黏菌亚纲Columellomycetidae,其下含有刺轴菌目Echinosteliales、绒泡菌目Physarales、发网菌目Stemonitales、碎皮菌目Clastodermatales和裂皮菌目Meridermatales. ...
... ).黏菌分类鉴定主要依靠子实体的形态特征,如基质层有无、柄的形态与质地、囊轴形态、孢丝有无与形态、孢子大小与纹饰等(李玉等 2008;Leontyev et al. 2019).自17世纪中叶起,菌物学工作者陆续在世界范围内开展了一系列黏菌资源的调查工作,迄今全球已被描述并认可的黏菌约1 000种(Lado 2005-2021).传统的黏菌分类系统将黏菌纲分为3亚纲6目(李玉等 2008),即鹅绒菌亚纲Ceratiomyxomycetidae鹅绒菌目Ceratiomyxales,腹黏菌亚纲Myxogastromycetidae刺轴菌目Echinosteliales、无丝菌目Liceales、团毛菌目Trichiales和绒泡菌目Physarales,发网菌亚纲Stemonitomycetidae发网菌目Stemonitales.晚近,Leontyev et al.(2019)结合分子系统学和形态分类学研究对黏菌分类系统做出较大调整,在保留主要的固有分类单元并遵循系统发育单系性的原则下,在黏菌纲中排除鹅绒菌目Ceratiomyxales,并且基于浅色孢子分支成立浅孢黏菌亚纲Lucisporomycetidae,其下含有无丝菌目Liceales、团毛菌目Trichiales、筛菌目Cribrariales和孔膜菌目Reticulariales;以子实体具有囊轴成立囊轴黏菌亚纲Columellomycetidae,其下含有刺轴菌目Echinosteliales、绒泡菌目Physarales、发网菌目Stemonitales、碎皮菌目Clastodermatales和裂皮菌目Meridermatales. ...
... ).自17世纪中叶起,菌物学工作者陆续在世界范围内开展了一系列黏菌资源的调查工作,迄今全球已被描述并认可的黏菌约1 000种(Lado 2005-2021).传统的黏菌分类系统将黏菌纲分为3亚纲6目(李玉等 2008),即鹅绒菌亚纲Ceratiomyxomycetidae鹅绒菌目Ceratiomyxales,腹黏菌亚纲Myxogastromycetidae刺轴菌目Echinosteliales、无丝菌目Liceales、团毛菌目Trichiales和绒泡菌目Physarales,发网菌亚纲Stemonitomycetidae发网菌目Stemonitales.晚近,Leontyev et al.(2019)结合分子系统学和形态分类学研究对黏菌分类系统做出较大调整,在保留主要的固有分类单元并遵循系统发育单系性的原则下,在黏菌纲中排除鹅绒菌目Ceratiomyxales,并且基于浅色孢子分支成立浅孢黏菌亚纲Lucisporomycetidae,其下含有无丝菌目Liceales、团毛菌目Trichiales、筛菌目Cribrariales和孔膜菌目Reticulariales;以子实体具有囊轴成立囊轴黏菌亚纲Columellomycetidae,其下含有刺轴菌目Echinosteliales、绒泡菌目Physarales、发网菌目Stemonitales、碎皮菌目Clastodermatales和裂皮菌目Meridermatales. ...
黏菌学的发展
1
2021
... 早期的黏菌生物学围绕黏菌生活史开展了大量研究,其中一些研究揭示了孢子萌发、黏变形体生长、原生质团行为等与所处的生态环境,包括温度、湿度、pH等一系列环境因素之间的关系(Madelin 1984),可以将这些研究归纳到个体生态学的范畴.Maimoni- Rodella & Gottsberger(1980)定量分析了巴西热带森林中的黏菌群落与基质类型、温度和降水量的关系,开启了黏菌的群落生态学研究.目前,绝大多数黏菌生态学研究首先通过子实体调查和分类鉴定,获得一定区域或生境内的黏菌物种组成与丰度信息;再应用数量生态学的分析手段,揭示黏菌群落的物种分布格局和影响因素等(Stephenson & Rojas 2017).这种基于子实体调查的方法不可避免地忽略了环境中一些无法发育为子实体的黏菌营养体(李玉等 2021),因此,许多研究者尝试将DNA分子标记和指纹图谱技术用于解析环境中的黏菌群落,如PCR-DGGE(Kamono & Fukui 2006;Kamono et al. 2009b)、PCR-T-RFLP(Hoppe & Schnittler 2015)等,标志着黏菌生态学研究已进入分子时代.当前,黏菌DNA条形码与高通量测序结合产生的扩增子高通量测序为黏菌生态学研究提供了新方法,通过环境基因组DNA提取、条形码基因PCR扩增、产物高通量测序和序列分类注释,已实现了不依赖子实体调查的群落定性、定量分析(Borg Dahl et al. 2018a). ...
黄连山自然保护区黏菌的物种多样性
1
2014
... 黏菌群落的时间动态一般由不同季节的湿热条件决定(Stephenson 1988;Takahashi & Hada 2012).基于野外采集和湿室培养研究热带地区的低海拔森林和农田生态系统发现,雨季黏菌的丰富度和多样性显著高于旱季(Tran et al. 2006;2008;Ko Ko et al. 2011).宋天鹏和陈双林(2014)在中国云南黄连山保护区较高海拔的亚热带森林的研究显示,雨季的黏菌多样性低于旱季,该研究通过湿室培养法收集黏菌子实体,反映出旱季的基物上存在更多黏菌休眠体.一些黏菌的发生具有季节性,造就了黏菌群落组成的季节性差异,如发网菌目黏菌多发生于雨季、绒泡菌目多发生于旱季(Ko Ko et al. 2011).日本温带森林凋落物栖生黏菌具有明显的季节发生规律,根据物种组成分为早期(4-5月)、中期(6-8月)和晚期(10-11月)3个阶段(Takahashi & Hada 2012).类似地,中国河南宝天曼保护区林中凋落物栖生黏菌群落在春、夏、秋3季也显著不同,基物类型及月平均气温与降水等气象因子是影响黏菌群落组成的主要因素(Gao et al. 2018). ...
鼎湖山自然保护区黏菌的物种组成和多样性
2
2016
... 热带、亚热带黏菌的早期研究仅限于零星报道.20世纪70年代,Farr(1976)系统整理了前人文献资料并记述了新热带区280种黏菌.在此基础上,Lado & Wrigley de Basanta(2008)重新梳理了分布于新热带地区的黏菌物种,共计431种.近年,研究人员陆续在我国热带和亚热带森林(闫淑珍等2012;Liu et al. 2013;魏滨等 2016;Gao et al. 2018;Li et al. 2021)、东南亚热带雨林开展了系统调查(Tran et al. 2006;2008;Ko Ko et al. 2010,2012,2013;Dagamac et al. 2011,2014,2017a;Novozhilov et al. 2017a,2020),极大地丰富了黏菌物种的地理分布和区系特征. ...
... 黏菌群落的垂直分布格局研究显示,黏菌的物种多样性与海拔总体呈负相关关系,但驱动这一分布格局的环境因子各不相同,例如,Stephenson et al.(2004)认为在厄瓜多尔热带云雾林中过高的湿度条件是高海拔样地黏菌多样性低的主要原因;Rojas & Stephenson(2007)研究发现pH是驱动可可岛黏菌多样性黏菌垂直分布的关键因素;魏滨等(2016)认为不同海拔梯度下的气流差异驱动了中国广东鼎湖山自然保护区黏菌垂直分布的形成;Rojas et al.(2016)选择植被类型均一的海拔梯度样带进行研究后发现,黏菌多样性和群落组成均与海拔不相关,认为黏菌的垂直分布格局应归因于不同海拔的植被差异. ...
中国热带黏菌的已知种类
1
2012
... 热带、亚热带黏菌的早期研究仅限于零星报道.20世纪70年代,Farr(1976)系统整理了前人文献资料并记述了新热带区280种黏菌.在此基础上,Lado & Wrigley de Basanta(2008)重新梳理了分布于新热带地区的黏菌物种,共计431种.近年,研究人员陆续在我国热带和亚热带森林(闫淑珍等2012;Liu et al. 2013;魏滨等 2016;Gao et al. 2018;Li et al. 2021)、东南亚热带雨林开展了系统调查(Tran et al. 2006;2008;Ko Ko et al. 2010,2012,2013;Dagamac et al. 2011,2014,2017a;Novozhilov et al. 2017a,2020),极大地丰富了黏菌物种的地理分布和区系特征. ...
小兴安岭两种林地的黏菌物种多样性
1
2019
... 自Panckow在1654年首次记录并描述粉瘤菌Lycogala epidendrum开始,尤其是Rostafinski在1873年建立第一个黏菌现代分类系统以来(Alexopoulos et al. 1996),黏菌工作者陆续在世界各地开展了大量的标本采集和经典分类的研究工作,出版了众多专著.但在很长一段时期内,研究区域主要集中于北半球的温带地区,其中以北美、欧洲和亚洲的温带森林生态系统最为突出(Stephenson et al. 2008),不仅有众多的论文,而且有许多地区志,包括北美的《The North American slime-moulds》和《The Mycetozoa of North America》,欧洲的《The Myxomycetes of Great Britain》《A monograph of the Mycetozoa— Being a descriptive catalogue of the species in the herbarium of the British Museum》《Mycological flora of European and Asian Russia. The slime molds》《A guide to temperate Myxomycetes》《Die Myxomyceten Deutschlands und des angrenzenden Alpenraumes unter besonderer Berücksichtigung Österreichs》《The Myxomycetes of Britain and Ireland. An identification handbook》和《Les Myxomycètes》,亚洲的《The Myxomycetes of Japan》《The Myxomycetes of India》《Taxonomy of the Indian Myxomycetes》《The myxomycete biota of Japan》和《中国真菌志·黏菌卷》等等.从已有调查结果来看,温带落叶阔叶林可能是地球上黏菌物种多样性最高的生态系统,如美国大雾山国家公园报道黏菌168种(Stephenson et al. 2001);俄罗斯锡霍特-阿林保护区报道黏菌158种(Novozhilov et al. 2017b);中国小兴安岭地区报道黏菌152种(赵凤云等 2019). ...