篮状菌属Talaromyces C.R. Benj.隶属于真菌界Fungi,子囊菌门Ascomycota,散囊菌纲Eurotiomycetes,散囊菌目Eurotiales,发菌科Trichocomaceae。篮状菌属物种无性阶段的繁殖结构为分生孢子梗(conidiophore),通常产生对称双轮生帚状枝(symmetrical biverticillate penicillus),其产孢细胞称为瓶梗(phialide),多为披针形(acerose),有些为安瓿形(ampuliform)。篮状菌属物种若产生有性阶段则其子囊果为裸囊壳(gymnothecium),壳内产生不定数目的球形至椭球形子囊,每个子囊含8个子囊孢子,无规则分散于子囊中。
篮状菌最初被当做青霉Penicillium Link来研究,如在Raper & Thom(1949)出版的《青霉手册》(《A Manual of Penicillia》)中,不论是否产生有性阶段而按照帚状枝分枝形态将青霉划分为4个组(section)和41个系(series),其中篮状菌被划分在青霉双轮对称组Penicillium section Biverticillata-Symmetrica Thom中。Benjamin(1955)依据当时的国际植物命名法规(International Code of Botanical Nomenclature,ICBN),给这类菌提出了一个有性型的属名,即篮状菌属Talaromyces,模式种为蠕形青霉P. vermiculatum Dangeard,即蠕形篮状菌T. vermiculatus (Dangeard) C.R. Benjamin。Pitt(1979)根据双重命名法(dual nomenclature),分别处理无性型和有性型,将只发现无性型的种归入青霉属,进而又将其分为4个亚属subgenus,即曲霉状亚属subgenus Aspergilloides Pitt、叉状亚属subgenus Furcatum Pitt、青霉亚属subgenus Penicillium和双轮亚属subgenus Biverticillium Pitt;将发现有性和/或无性阶段的种分别归入正青霉属Eupenicillium (F. Ludw.)和篮状菌属。其中只发现无性型的且具有Penicillium section Biverticillata-Symmetrica分类学性状的种被放在subgenus Biverticillium Pitt中,将产生裸囊壳的种放在篮状菌属中。后来的分子种系发生学(phylogenetics,Gr. Phyle=tribe,genesis=birth)的研究,如根据rDNA ITS1-5.8S- ITS2(ITS)、β-微管蛋白基因(β-tubulin gene,BenA)和钙调蛋白基因(calmodulin gene,CaM)序列的分析显示双轮亚属的种与其他亚属的青霉种分属不同的演化支(clade)而与篮状菌属处于同一个演化支(clade)(Wang & Zhuang 2007;Houbraken & Samson 2011;Samson et al. 2011)。2012年的《国际藻类、真菌和植物命名法规》(墨尔本法规)[International Code of Nomenclature for algae, fungi, and plants (Melbourne Code)]取消了真菌的双重命名系统(dual naming system),明确规定一种真菌只有一个名称(one fungus,one name)。因此,Talaromyces成为上述青霉属双轮亚属和篮状菌属物种的合法属名(McNeill et al. 2012)。
Samson et al.(2011)承认了71个Talaromyces物种,Yilmaz et al.(2014)根据BenA、CaM、ITS和Rpb2(RNA多聚酶Ⅱ第二大亚基,DNA-dependent RNA polymerase II the second largest subunit)将已经发现的88种篮状菌分为7个组:杆孢篮状菌组section Bacillispori 6种、螺旋篮状菌组section Helici 7种、岛篮状菌组section Islandici 15种、紫篮状菌组section Purpurei 10种、近膨篮状菌组section Subinflati 2种、篮状菌组section Talaromyces 36种和糙孢篮状菌组section Trachispermi 12种。最近Sun et al.(2020)又增加了一个组,即细梗篮状菌组section Tenues 1种。篮状菌组是篮状菌属的最大组,截至本文投稿时全球该组已经报道72个种,我国报道了26个种(Tzean et al. 1994;孔华忠和王龙 2007;Chen et al. 2016;Wang et al. 2016a,2016b;Wang et al. 2017;Su & Niu 2018;Jiang et al. 2018;陈晗等2020;孙剑秋等2020;Sun et al. 2020;王龙等 2020)。在系统调查我国篮状菌物种资源并编研“中国篮状菌志”的研究工作中,我们从5个省区的土壤中分离得到10株篮状菌,经鉴定分属6个种,即艾米斯托克篮状菌T. amestolkiae N. Yilmaz, Houbraken, Frisvad & Samson、暗玫瑰篮状菌T. atroroseus N. Yilmaz, Frisvad, Houbraken & Samson、暗绿篮状菌T. fuscoviridis Yilmaz, Visagie & Samson、肯德里克篮状菌T. kendrickii Yilmaz, Visagie, Seifert & Frisvad、斯托尔篮状菌T. stollii N. Yilmaz, Houbraken, Frisvad & Samson和多样篮状菌T. versatilis Bridge & Buddie。其中T. atroroseus属于section Trachispermi,其余5种均属于section Talaromyces,除T. amestolkiae外,T. fuscoviridis、T. kendrickii、T. stollii和T. versatilis为我国新记录种。
1 材料与方法
1.1 样品采集和分离
土壤样品采自我国贵州、湖南、黑龙江、江西、北京等地,取表层土下面富含腐殖质的土壤约25g置于无菌塑料袋中封好。样品分离采用改进的Malloch(1981)倍比稀释涂布平皿法(王龙等2020),分离得到的10株篮状菌,经鉴定后将每个种的代表菌株保存于中国普通微生物菌种保藏中心(CGMCC)(表1)。
表1 用于分子种系学分析的篮状菌组48种58株菌及其3个遗传标记(以暗玫瑰篮状菌为外群)
Table 1
| 物种 Species | 菌株 Strainsa, b | 来源 Sources | 遗传标记 Genetic markers | ||
|---|---|---|---|---|---|
| BenA | CaM | ITS | |||
| T. aculeatus | NRRL 2129T= CBS 289.48 | 美国织物 Textile, USA | KF741929 | KF741975 | KF741995 |
| T. adpressus | CGMCC 3.18211T= CBS 140620 | 中国北京室内空气 Indoor air, Beijing, China | KU866844 | KU866741 | KU866657 |
| T. amestolkiae | CBS 132696T= DTO 179F5 | 南非开普敦室内尘土 House dust, Cape Town, South Africa | JX315623 | KF741937 | JX315660 |
| 3.15821=14049 | 中国贵州省黄果树瀑布景区土壤 Soil, Huangguoshu Waterfall, Guizhou, China | MT892944 | MT892950 | MT883346 | |
| 12686 | 中国浙江农业大学茶园土壤 Soil, field of Camellia sinensis, Zhejiang Agricultural University, China | MT892943 | MT892949 | MT883345 | |
| HL72 | 中国黑龙江五大连池土壤 Soil, Wudalianchi, Heilongjiang, China | MT892945 | MT892951 | MT883347 | |
| T. angelicus | KACC 46611T | 韩国平昌干燥当归根 Dried root of Angelica gigas, Pyeong chang, Gangwon-do, Republic of Korea | KF183640 | KJ885259 | KF183638 |
| T. apiculatus | CBS 312.59T | 日本土壤 Soil, Japan | KF741916 | KF741950 | JN899375 |
| T. argentinensis | NRRL 28750T | 加纳塔佛土壤 Soil, Tafo, Ghana | MH792917 | MH792981 | MH793045 |
| T. australis | CBS 137102T | 澳大利亚牧场土壤 Soil under pasture, Australia | KF741922 | KF741971 | KF741991 |
| T. beijingensis | CGMCC 3.18200T= CBS 140617 | 中国北京室内空气 Indoor air, Beijing, China | KU866837 | KU866733 | KU866649 |
| T. californicus | NRRL 58168T | 美国加利福尼亚空气 Air, California, USA | MH792928 | MH792992 | MH793056 |
| T. cnidii | KACC 46617T | 韩国堤川干燥川芎根 Dried roots of Cnidium officinale, Jecheon, Chungbuk, Republic of Korea | KF183641 | KJ885266 | KF183639 |
| T. derxii | CBS 412.89T | 日本仓敷耕作土壤 Cultivated soil, Kurashiki, Japan | JX494306 | KF741959 | JN899327 |
| T. dimorphus | AS3.15692T | 中国海南尖峰岭土壤 Soil, Jianfengling Forest Park, Hainan, China | KY007111 | KY007103 | KY007095 |
| T. duclauxii | CBS 322.48T | 法国帆布 Canvas, France | JX091384 | KF741955 | JN899342 |
| T. euchlorocarpius | DTO 176I3T | 日本横滨土壤 Soil, Yokohama, Japan | KJ865733 | KJ885271 | AB176617 |
| T. flavovirens | CBS 102801T | 西班牙赫罗纳栓皮栎落叶 Quercus suber leaf litter, Selva de Mar, Gerona, Catalonia, Spain | JX091376 | KF741933 | JN899392 |
| T. flavus | CBS 310.38T | 新西兰未知来源 Unknown source, New Zealand | JX494302 | KF741949 | JN899360 |
| T. francoae | CBS 113134T | 哥伦比亚亚马逊厚隔香属植物落叶 Leaf litter in Pseudomonotes tropenbosii forest in Peña Roja, Dept. Amazonas, Colombia | KX011489 | KX011501 | KX011510 |
| T. funiculosus | CBS 272.86T | 印度葫芦 Lagenaria vulgaris, India | JX091383 | KF741945 | JN899377 |
| T. fuscoviridis | CBS 193.69T | 尼德兰土壤 Soil, the Netherlands | KF741912 | KF741942 | KF741979 |
| AS3.15876= JX6-6 | 中国江西庐山土壤 Soil, Lushan, Jiangxi, China | MK837943 | MK837951 | MK837959 | |
| T. fusiformis | CGMCC 3.18210T= CBS 140637 | 中国北京室内空气 Indoor air, Beijing, China | KU866843 | KU866740 | KU866656 |
| T. galapagensis | CBS 751.74T= NRRL 13068 | 厄瓜多尔龟岛美登木下土壤 Soil beneath Maytenus obovata, Galapagos Island, Ecuador | JX091388 | KF741966 | JN899358 |
| T. intermedius | CBS 152.65T | 英国诺丁汉沼泽土壤 Alluvial pasture and swamp soil, Nottingham, UK | JX091387 | KJ885290 | JN899332 |
| T. kendrickii | CBS 136666T= IBT13593 | 加拿大森林土壤 Forest soil, Canada | KF741921 | KF741967 | KF741987 |
| CBS 136669= IBT14128 | 尼日利亚土壤 Soil, Nigeria | KF741925 | KF741968 | KF741988 | |
| 3.15849=HL320 | 中国黑龙江伊春凉水自然保护区土壤 Soil, Liangshui Nature Reserve, Yichun, Heilongjiang, China | MT892947 | MT892953 | MT883349 | |
| 3.15852=AC151 | 中国北京顺义土壤 Soil, Shunyi, Beijing, China | MT892948 | MT892954 | MT883350 | |
| T. lentulus | AS3.15689T | 中国山东东营土壤 Soil, Dongying, Shandong, China | KY007104 | KY007096 | KY007088 |
| T. liani | CBS 225.66T= NRRL 3380 | 中国土壤 Soil, China | JX091380 | KJ885257 | JN899395 |
| T. louisianensis | NRRL 35823T | 美国路易斯安娜空气 Air, Louisiana, USA | MH792924 | MH792988 | MH793052 |
| T. macrosporus | CBS 317.63T | 南非斯泰伦博斯苹果汁 Apple juice, Stellenbosch, South Africa | JX091382 | KF741952 | JN899333 |
| T. mae | AS3.15690T | 中国上海东平国家森林公园土壤 Soil, Dongping National Forest Park, Shanghai, China | KY007106 | KY007098 | KY007090 |
| T. mangshanicus | AS3.18013T | 中国湖南郴州莽山国家森林公园土壤 Soil, Mangshan National Forest Park, Chenzhou, Hunan, China | KX447530 | KX447528 | KX447531 |
| T. marneffei | CBS 388.87T | 越南竹鼠 Bamboo rat (Rhizomys sinensis), Vietnam | JX091389 | KF741958 | JN899344 |
| T. panamensis | CBS 128.89T | 巴拿马巴罗科罗拉多岛土壤 Soil, Barro Colorado Island, Panama | HQ156948 | KF741936 | JN899362 |
| T. pinophilus | CBS 631.66T | 法国聚氯乙烯 PVC, France | JX091381 | KF741964 | JN899382 |
| T. primulinus | CBS 321.48T | 美国未知来源 Unknown source, USA | JX494305 | KF741954 | JN899317 |
| T. purpureogenus | CBS 286.36T | 日本米曲霉寄生菌 Parasitic on a culture of Aspergillus oryzae, Japan | JX315639 | KF741947 | JN899372 |
| T. qii | AS3.15414T= CBS 139515 | 中国西藏墨脱土壤 Soil, Motuo County, Tibet, China | KP765380 | KP765382 | KP765384 |
| T. rapidus | CBS 142382T | 美国俄亥俄人肺泡灌洗液 Human bronchoalveolar lavage, Ohio, USA | LT559087 | LT795600 | LT558970 |
| T. ruber | CBS 132704T | 英国航空器油箱 Aircraft fuel tank, UK | JX315629 | KF741938 | JX315662 |
| T. rubicundus | CBS 342.59T | 美国乔治亚土壤 Soil, Georgia, USA | JX494309 | KF741956 | JN899384 |
| T. sayulitensis | NRRL 62185T | 美国南卡罗莱纳玉米 Corn, South Carolina, USA | MH792950 | MH793014 | MH793077 |
| T. siamensis | CBS 475.88T | 泰国南邦府森林土壤 Forest soil, Lampang, Thailand | JX091379 | KF741960 | JN899385 |
| T. stollii | CBS 408.93T | 尼德兰艾滋病人 AIDS patient, the Netherlands | JX315633 | JX315646 | JX315674 |
| AS3.16017= JN1-1 | 中国山西阳泉孟县腐殖酸 Humic acid, Mengxian, Yangquan, Shanxi, China | MW025969 | MW053682 | MW053684 | |
| HH1-1 | 中国黑龙江黑河锦河峡谷土壤 Soil, Jinhe Grand Canyon, Heihe, Heilongjiang, China | MW025968 | MW053681 | MW053683 | |
| T. thailandensis | CBS 133147T | 泰国土壤 Soil, Thailand | JX494294 | KF741940 | JX898041 |
| T. veerkampii | CBS 500.78T | 哥伦比亚维拉维森西奥土壤 Soil, Dep. de Meta, Municipio de Villavicencio, Colombia | KF741918 | KF741961 | KF741984 |
| T. verruculosus | NRRL 1050T= CBS 388.48 | 美国德克萨斯土壤 Soil, Texas, USA | KF741928 | KF741974 | KF741994 |
| T. versatilis | IMI 134755T= CBS 140377 | 英国锡莱研究所来源未知 Unknown source, Shirley Institute, UK | KC992270 | MN969319 (DTO 326-B7) | KC962111 |
| AS3.15853= 3708 | 中国未知来源 Unknown source, China | MK837944 | MK837952 | MK837960 | |
| T. viridis | CBS 114.72T= NRRL 5575 | 澳大利亚土壤 Soil, Australia | JX494310 | KF741935 | AF285782 |
| T. viridulus | CBS 252.87T | 澳大利亚新南威尔士土壤 Soil, New South Wales, Australia | JX091385 | KF741943 | JN899314 |
| T. xishaensis | AS3.17995T | 中国海南三沙永兴岛土壤 Soil, Yongxing Island, Sansha Hainan, China | KU644581 | KU644582 | KU644580 |
| T. atroroseus | HR11-1 | 中国北京怀柔皇后镇土壤 Soil, Queen’s Town, Huairou District, Beijing, China | MT892946 | MT892952 | MT883348 |
注:a模式菌株用“T”标出,b新记录种菌株和序列用粗体表示
Note: a Ex-type strains are indicated with “T”, b strains and sequences of the new records are indicated in boldface.
1.2 形态学研究方法
培养性状研究采用查氏酵母精琼脂(Czapek yeast autolysate agar,CYA)分别于25℃、37℃、5℃和麦芽精琼脂(5% malt extract agar,MEA)于25℃培养7d后观察、描述和照相。菌落颜色的描述参照Ridgway(1912)的色谱。显微结构研究采用在MEA 25℃培养7d的产孢结构做显微镜载片观察、照相和描述(Pitt 1979;Samson et al. 2010)。
1.3 PCR扩增和测序
核基因组DNA的提取参考Wang & Zhuang(2004)的方法,扩增BenA、CaM和ITS的引物分别参考Glass & Donaldson(1995)、Wang(2012)、White et al.(1990)的方法。PCR扩增反应在无菌的0.2mL薄壁平盖Eppendorf管中进行,20µL反应体系含有基因组DNA 1.0µL,正向和反向引物(10µmol/L)各0.5µL,双蒸水8µL,2×PCR扩增缓冲液(0.05U/µL Taq polymerase,4mmol/L MgCl2,0.4mmol/L dNTPs)10µL。PCR程序为94℃ 3min,然后共进行30个温度循环:94℃变性30s,50℃退火30s,72℃延伸30s,最后在72℃延伸5min。PCR产物各取5µL与5µL的100bp DNA ladder用2.0%的琼脂糖凝胶(agarose gel)在80V电压下电泳15min,再用0.5g/mL的溴乙锭(ehidium bromide,EB)染色10min后在波长365和254nm的紫外灯下观察。显示单一、明亮扩增区段长度条带的PCR扩增产物(BenA约400bp,CaM约700bp,ITS约600bp)用ABI3730(Applied Biosystems,Drive Foster City,CA,USA)进行双向直通测序。
1.4 分子种系学分析
原始序列用生物学软件Bioedit 7.0.9(1999)进行人工校对、编辑,得到准确无误的全区段序列后提交到GenBank并用于种系学分析。选择篮状菌组48个物种的模式菌株和代表菌株49株以及本研究的10株共59株篮状菌的BenA、CaM和ITS序列链接成组合序列,其中以糙孢篮状菌组的T. atroroseus HR11-1作为外群(表1),用MEGA 6(2013)进行对位排列(alignment)并编辑修剪后做成序列矩阵,然后用最大似然法(maximum likelihood,ML)分析并采用自展法(bootstrap)进行1 000次重复评估各分支的可靠性,其中空格(gap)选择“partial deletion”(Hall 2013);另外,该序列矩阵还采用贝叶斯法(bayes inference,BI)对各分支进行后验概率分析(posterior probability,PP)(Ronquist et al. 2012)。
2 结果与分析
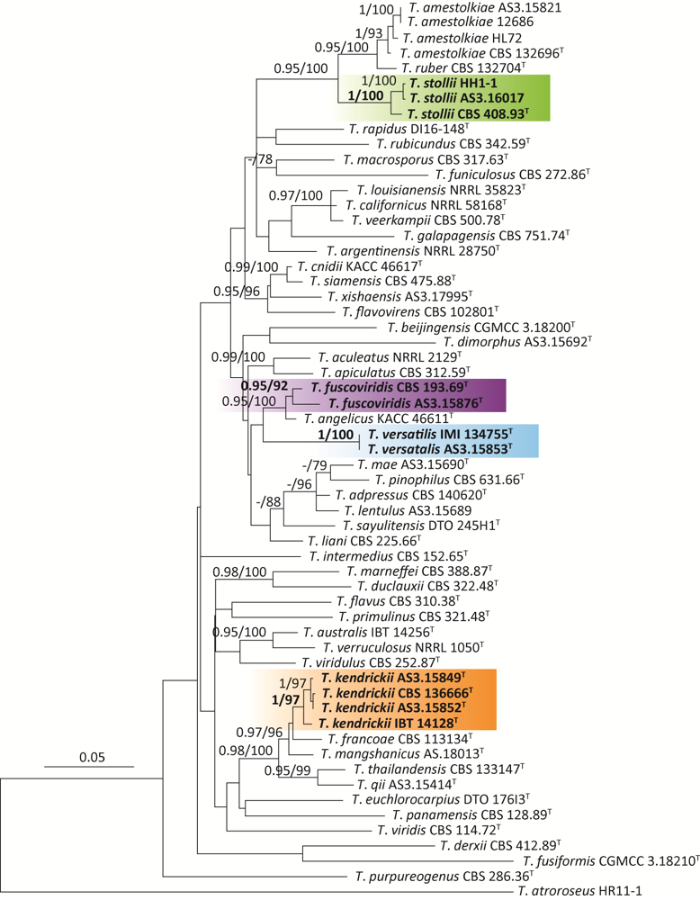
BenA-CaM-ITS的组合序列矩阵共1 261个位点(site),分子种系学分析显示菌株AS3.15876=JX6-6与T. fuscoviridis的模式菌株CBS 193.69同在一个分支,支持率为92%,BI后验概率为0.95;菌株AS3.15849=HL320和AS3.15852=AC151与T. kendrickii的模式菌株CBS 136666同在一个分支,支持率为100%,BI后验概率为1;菌株AS3.16017=JN1-1和HH1-1与T. stollii的模式菌株CBS 408.93同在一个分支,支持率为100%,BI后验概率为1;菌株AS3.15853=3708与T. versatilis的菌株IMI 134755同在一个分支,支持率为100%,BI后验概率为1。结合形态学和分子种系学分析确认这些菌株的鉴定准确无误,参考我国已报道的篮状菌物种,确定这4个种均为我国新记录种(图1-图5)。
图1
图1
基于BenA-CaM-ITS的ML系统发育树
BI后验概率≥0.95和支持率≥70%标注在分支节点处,T表示模式菌株,粗体表示新记录种. 标尺=0.05每核苷酸替代率
Fig. 1
ML phylogram inferred from concatenated BenA-CaM-ITS partial sequences.
Posterior probabilities of BI over 0.95 and percentages over 70% derived from 1 000 replicates are indicated at the nodes, T indicates ex-type strains, the species new to China are indicated in boldface. Bar=0.05 substitutions per nucleotide position.
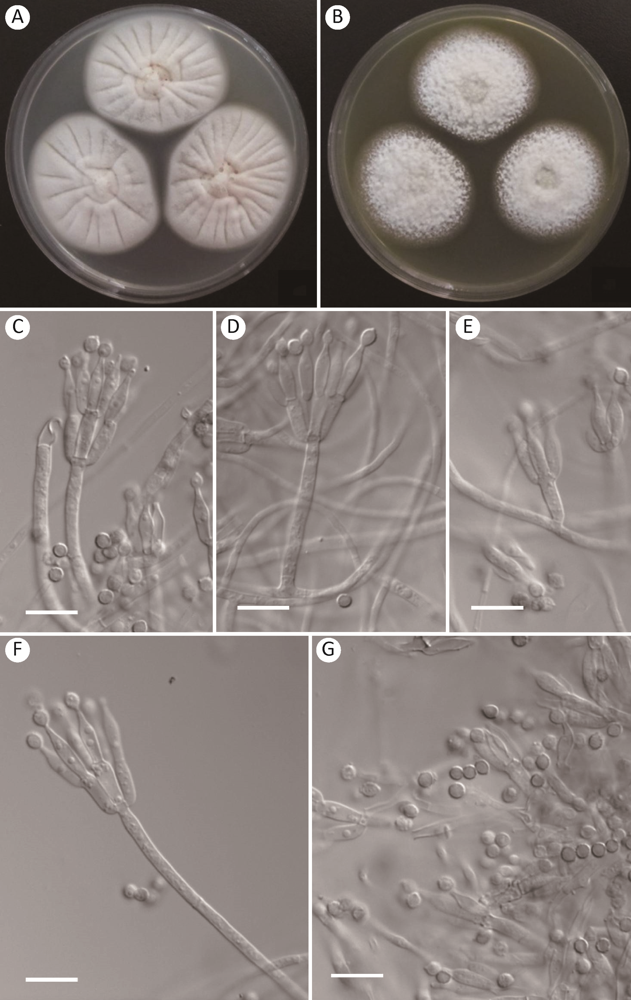
图2
图2
暗绿篮状菌Talaromyces fuscoviridis AS3.15876的形态学性状
A,B:在CYA和MEA上25℃、7d的菌落;C-F:分生孢子梗;G:分生孢子. 标尺=10μm
Fig. 2
Morphology of Talaromyces fuscoviridis AS3.15876.
A, B: Colonies on CYA and MEA at 25°C after 7d; C-F: Conidiophores; G: Conidia. Scale bars=10μm.
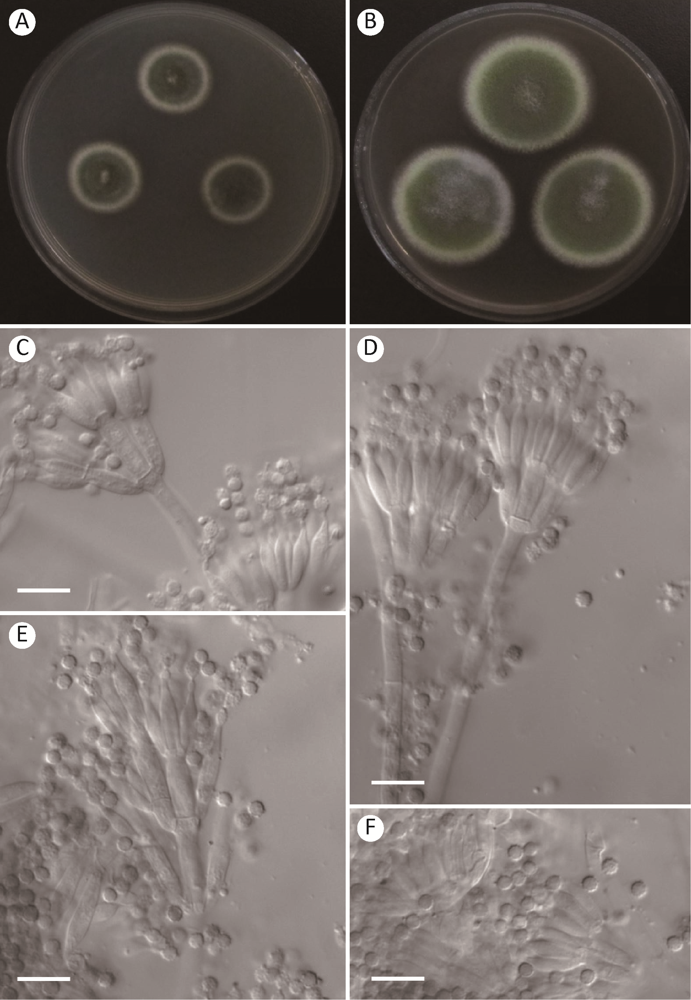
图3
图3
肯德里克篮状菌Talaromyces kendrickii AS3.15849的形态学性状
A,B:在CYA和MEA上25℃、7d的菌落;C-E:分生孢子梗;F:分生孢子. 标尺=10μm
Fig. 3
Morphology of Talaromyces kendrickii AS3.15849.
A, B: Colonies on CYA and MEA at 25°C after 7d; C-E: Conidiophores; F: Conidia. Scale bars=10μm.
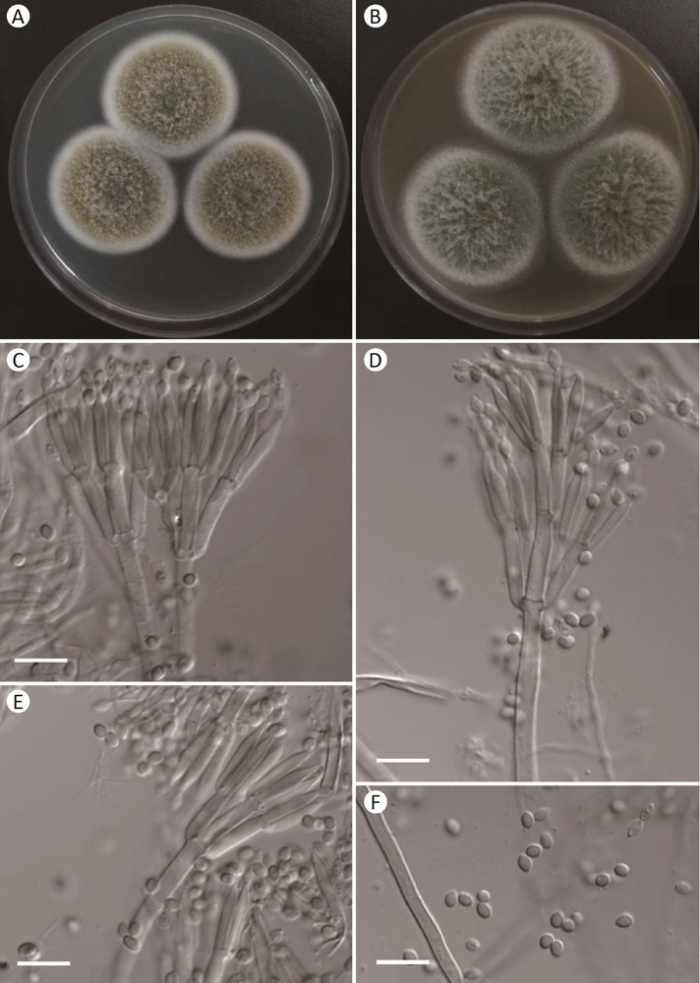
图4
图4
斯托尔篮状菌Talaromyces stollii AS3.16017的形态学性状
A,B:在CYA和MEA上25℃、7d的菌落;C-E:分生孢子梗;F:分生孢子. 标尺=10μm
Fig. 4
Morphology of Talaromyces stollii AS3.16017.
A, B: Colonies on CYA and MEA at 25°C after 7d; C-E: Conidiophores; F: Conidia. Scale bars=10μm.
图5
图5
多样篮状菌Talaromyces versatilis AS3.15853的形态学性状
A,B:在CYA和MEA上25℃、7d的菌落;C-F:分生孢子梗;G:菌丝绳;H:分生孢子. 标尺=10μm
Fig. 5
Morphology of Talaromyces versatilis AS3.15853.
A, B: Colonies on CYA and MEA at 25°C after 7d; C-F: Conidiophores; G: The hyphal funiculus; F: Conidia. Scale bars=10μm.
2.1 暗绿篮状菌 图2
Talaromyces fuscoviridis Visagie, N. Yilmaz & Samson, Mycoscience 56: 492, 2015.
在查氏酵母精琼脂(CYA)上25℃、7d,菌落直径43-45mm,较薄,具较多辐射状沟纹,边缘完整;质地绒状兼短绳状;分生孢子结构无;菌丝体白色夹杂淡黄色;渗出液少量,呈珊瑚红色coral pink(R. Pl. XIII)或无色;可溶性色素无;菌落背面浅黄色,近于pinkish buff(R. Pl. XXIX)。
在麦芽精琼脂(MEA)上25℃、7d,菌落直径40-41mm,稍厚,中央凹陷,边缘于培养基内,完整;质地短絮状兼绳状;分生孢子结构稀少,近于淡烟灰色pale smoke gray(R. Pl. XLVI);菌丝体白色夹杂浅黄色,近于cream color(R. Pl. XVI);渗出液无;可溶性色素无;菌落背面呈暗绿色,近于darkyellowish green to ackermann’s green(R. Pl. XVIII)。
在CYA上37℃、7d,菌落直径约12mm,稍厚,无规则沟纹少,边缘完整;质地绒状;分生孢子结构无;菌丝呈脏粉色,近于pale congo pink(R. Pl. XXVIII);渗出液无;可溶性色素多,深粉红色,近于old rose(R. Pl. XIII);菌落背面呈深红色,近于Nopal red(R. Pl. I)。
在CYA上5℃、7d,未生长。
分生孢子梗产生于表面菌丝和气生菌丝,孢梗茎50-150×2.5-3μm,壁光滑;帚状枝双轮生兼单轮生;梗基每轮2-4个,排列紧密,10-11×2.5-3μm;瓶梗安瓿形,排列不紧密,每轮2-4个,10-11×2.5-3μm;分生孢子椭球形至近球形,3-3.5(-4)μm,壁厚,光滑至稍粗糙。
分布和基物:江西庐山土壤(JX6-6= AS3.15876)。
注:本种生长较快,MEA菌落背面呈特征性暗绿色,产生短絮状兼绳状菌落,分生孢子稀疏,灰绿色,在37℃生长局限;帚状枝双轮生兼单轮生,排列紧密,分生孢子球形至近球形,壁厚,光滑。
2.2 肯德里克篮状菌 图3
Talaromyces kendrickii Visagie, N. Yilmaz, Seifert & Frisvad, Mycoscience 56: 493, 2015.
在查氏酵母精琼脂(CYA)上25℃、7d,菌落直径22-25mm,较薄,平坦,边缘于培养基内,整齐;质地绒状;分生孢子大量,灰橄榄色grayish olive(Ridgway Pl. XLVI);菌丝体在边缘呈白色,在近边呈硫磺色sulphur yellow(Ridgway Pl. V);渗出液无;可溶性色素无;菌落背面呈烧土褐色burnt sienna(Ridgway Pl. III)。
在麦芽精琼脂(MEA)上25℃、7d,菌落直径35-38mm,较薄,平坦,边缘于培养基内,整齐;质地绒状;分生孢子大量,草绿色grass green(Ridgway Pl. VI);菌丝体呈浅绿黄色light viridine yellow(Ridgway Pl. V);渗出液无;可溶性色素无;菌落背面呈赭色,但中央呈淡橙红色。
在CYA上37℃、7d,未生长。
在CYA上5℃、7d,未生长。
分生孢子梗产生于表面菌丝,孢梗茎200-400×3-4μm,壁光滑;帚状枝双轮生、单轮生兼不规则生;梗基每轮4-8个,排列
不紧密,10-15×2.5-4μm;瓶梗安瓿形,排列不紧密,每轮4-6个,10-12×2-3μm;分生孢子近球形至椭球形,2.5-3.5×2-3μm,壁粗糙。
分布和基物:黑龙江伊春凉水自然保护区土壤(HL320=AS3.15849);北京顺义土壤(AC151= AS3.15852)。
注:该种生长适中,产生绒状菌落和大量草绿色分生孢子,帚状枝双轮生和不规则生,排列不紧密,瓶梗安瓿形,分生孢子近球形至椭球形,壁粗糙。
2.3 斯托尔篮状菌 图4
Talaromyces stollii N. Yilmaz, Houbraken, Frisvad & Samson, Persoonia 29: 52, 2012.
在查氏酵母精琼脂(CYA)上25℃、7d,菌落直径39-42mm,较薄,平坦,边缘于培养基内,流苏状;质地绒状,表面覆盖大量絮状菌丝;分生孢子大量,豆绿色pea green (R. Pl. XLVII);菌丝体在边缘呈白色,其余夹杂淡橄榄红色olive lake(R. Pl. XVI);渗出液无;可溶性色素无;菌落背面中央呈红色,边缘颜色变浅至淡赭鲑色pale ochraceous- salmon(R. Pl. XV)。
在麦芽精琼脂(MEA)上25℃、7d,菌落直径47-49mm,稍厚,平坦,边缘于培养基内,流苏状;质地绒状且表面覆盖大量白色絮状菌丝;分生孢子大量,豆绿色至鼠尾草绿色pea green to sage green(R. Pl. XLVII);菌丝体呈白色;渗出液无;可溶性色素无;菌落背面呈赭色。
在CYA上37℃、7d,菌落直径28-30mm,类似于CYA上25℃、7d。
在CYA上4℃、7d,不生长。
分生孢子梗产生于基质,孢梗茎100-150 (-200)×3-5μm,壁光滑;帚状枝双轮生兼三轮生;梗基每轮3-6个,排列紧密,10-14× 2.5-3.5μm;瓶梗披针形,排列紧密,每轮4-6个,11-15×2-3μm;分生孢子椭球形,2.5-4×2-3μm,壁光滑。
分布和基物:黑龙江黑河锦河大峡谷(HH1-1),山西阳泉孟县腐殖酸(JN1-1= AS3.16017)。
注:该种生长很快,在37℃生长正常,形成绒状兼絮状菌落,分生孢子大量,豆绿色,帚状枝双轮生和三轮生,排列紧密,瓶梗典型披针形,分生孢子椭球形,壁光滑。
2.4 多样篮状菌 图5
Talaromyces versatilis P.F. Cannon, Bridge & Buddie, Index Fungorum 26: 1, 2013.
在查氏酵母精琼脂(CYA)上25℃、7d,菌落直径约50mm,较薄,辐射状及同心环状沟纹少量,边缘于培养基内,完整;质地绒状,中部浸润状,呈砖红色;分生孢子结构无;菌丝体在边缘呈白色,在中部夹杂砖红色;砖红色渗出液大量,浸润于菌落中部,使得中部菌丝体呈砖红色;可溶性色素无;反面棕红色。
在麦芽精琼脂(MEA)上25℃、7d,菌落直径50-52mm,较薄,平坦,边缘于培养基内,完整;质地短絮状,表面覆盖菌丝绳长约3mm;分生孢子结构稀少,浅灰橄榄色light grayish olive (R. Pl. XLVI);菌丝体脏粉色,近于pinkish vinaceous(R. Pl. XXVII);渗出液无色;可溶性色素无;反面粉红色,近于orange vinaceous(R. Pl. XXVII)。
在CYA上37℃、7d,菌落直径约20-21mm,其他性状类似于CYA 25℃、7d。
在CYA上5℃、7d,未生长。
分生孢子梗产生气生菌丝和绳状菌丝,孢梗茎30-60(-120)×2-3μm,壁光滑;帚状枝双轮生兼单轮生;梗基每轮2-6个,排列不紧密,9-15×2-3μm;瓶梗安瓿形至圆柱形,排列紧密,每轮2-4个,8-12×2-3μm;分生孢子近球形,2.5-3×2-3μm,壁光滑。
分布和基物:安徽黄山土壤(AS3.15853= 3708)。
注:生长较快,形成短絮状兼绳状菌落,在37℃生长良好,分生孢子浅灰绿色,菌丝体呈砖红色至脏粉色,分生孢子梗较细,帚状枝双轮生兼单轮生,排列不紧密,瓶梗安瓿形,分生孢子近球形至椭球形,壁光滑。
3 讨论
Talaromyces fuscoviridis属于稀有种(rare species),但我们推测其分布应该比较广泛,因为该种在2015年建立后到目前GenBank只记录了6个株菌的序列,其在欧洲、亚洲、北美洲和南美洲均有报道(Peterson & Jurjevic 2019)。可能因为该物种在土壤真菌群落中较少而不容易分离到,本研究采用改进的土壤样品稀释涂布平皿法(王龙等2020)成功分离到该物种。我国的T. fuscoviridis菌株AS3.15876与模式菌株CBS 193.69在菌落形态上有一定差别,比如,该菌株生长快速,分生孢子稀疏,而模式菌株生长较慢,分生孢子适量。但菌株AS3.15876在MEA背面呈现与模式菌株完全相同的特征性暗绿色,而且它们的显微结构几乎完全相同,比如其分生孢子梗均发生于表面菌丝和气生菌丝,帚状枝为双轮生、单轮生,分生孢子均为椭球形至近球形,壁光滑至稍粗糙(Visagie et al. 2015)。分子种系学分析显示该菌株与T. fuscoviridis的模式菌株CBS 193.69同在一个分支且具有显著的高支持率(图1)。
Talaromyces kendrickii同样也应该属于稀有种但分布比较广泛。该种在2015年建立后到目前GenBank只记录7株菌的序列,但其在北美洲、非洲、欧洲和澳洲均有报道(Houbraken et al. 2020)。发现于我国的T. kendrickii这两株菌与模式菌株在菌落形态上存在一些差别。比如我国菌株均产生大量草绿色分生孢子,菌丝体颜色为黄绿色,但模式菌株CBS 136666的分生孢子稀疏,菌丝体颜色为浅粉色。但它们在显微结构上几乎完全相同,例如均产生双轮生和单轮生及不规则生帚状枝,排列不紧密,瓶梗安瓿形,分生孢子近球形至椭球形,壁粗糙(Visagie et al. 2015)。分子种系学分析也显示该菌株与T. kendrickii的模式菌株IBT 14128同在一个分支且具有显著的高支持率(图1)。
Talaromyces stollii是常见种(abundant species)且分布广泛,在世界范围内均有报道(Houbraken et al. 2020)。发现于我国的3个菌株的培养性状和显微性状几乎与模式菌株CBS 132696完全相同(Yilmaz et al. 2012)。该种与T. amestolkiae在菌落形态上很相似,依据形态学鉴定时容易混淆,尤其是其生长速度和分生孢子颜色非常相近。通过以下几方面的特征可以区分:T. stollii的菌落虽为绒状,但表面覆盖大量絮状菌丝而形成绒兼絮状菌落,而T. amestolkiae的菌落为典型绒状,只在CYA上有稀疏的絮状菌丝;在显微结构上,T. stollii产生的帚状枝排列紧密,常见三轮生的帚状枝,其瓶梗为典型披针形,而T. amestolkiae的帚状枝排列不紧密,偶见不规则的帚状枝,其瓶梗为安瓿形。
Talaromyces versatilis可能属于稀有种、狭域种,该种在2013年建立后到目前GenBank中只有6株菌的序列且只在欧洲和亚洲报道(Heo et al. 2019)。我国的T. versatilis菌株AS3.15853与模式菌株IMI 134755在菌落形态上基本一致,只是分生孢子产生较少,另外该菌株能形成菌丝绳,而模式菌株不形成菌丝绳。在显微结构上,除了该菌株产生双轮生和单轮生帚状枝,而模式菌株不形成单轮生帚状枝外,其他特征均与模式菌株相同。
参考文献
Ascocarps of Aspergillus and Penicillium
DOI:10.1080/00275514.1955.12024485 URL [本文引用: 1]
Talaromyces versatilis Bridge & Buddie, sp. nov
New Talaromyces species from indoor environments in China
DOI:10.1016/j.simyco.2016.11.003 URL [本文引用: 1]
Current taxonomy of Talaromyces and three new Chinese records
Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes
DOI:10.1128/aem.61.4.1323-1330.1995 URL [本文引用: 1]
Building phylogenetic trees from molecular data with MEGA
DOI:10.1093/molbev/mst012 URL [本文引用: 1]
Diversity of Aspergillus, Penicillium and Talaromyces species isolated from freshwater environments in Korea
DOI:10.1080/12298093.2019.1572262 URL [本文引用: 1]
Phylogeny of Penicillium and the segregation of Trichocomaceae into three families
DOI:10.3114/sim.2011.70.01
PMID:22308045
[本文引用: 1]

Species of Trichocomaceae occur commonly and are important to both industry and medicine. They are associated with food spoilage and mycotoxin production and can occur in the indoor environment, causing health hazards by the formation of β-glucans, mycotoxins and surface proteins. Some species are opportunistic pathogens, while others are exploited in biotechnology for the production of enzymes, antibiotics and other products. Penicillium belongs phylogenetically to Trichocomaceae and more than 250 species are currently accepted in this genus. In this study, we investigated the relationship of Penicillium to other genera of Trichocomaceae and studied in detail the phylogeny of the genus itself. In order to study these relationships, partial RPB1, RPB2 (RNA polymerase II genes), Tsr1 (putative ribosome biogenesis protein) and Cct8 (putative chaperonin complex component TCP-1) gene sequences were obtained. The Trichocomaceae are divided in three separate families: Aspergillaceae, Thermoascaceae and Trichocomaceae. The Aspergillaceae are characterised by the formation flask-shaped or cylindrical phialides, asci produced inside cleistothecia or surrounded by Hülle cells and mainly ascospores with a furrow or slit, while the Trichocomaceae are defined by the formation of lanceolate phialides, asci borne within a tuft or layer of loose hyphae and ascospores lacking a slit. Thermoascus and Paecilomyces, both members of Thermoascaceae, also form ascospores lacking a furrow or slit, but are differentiated from Trichocomaceae by the production of asci from croziers and their thermotolerant or thermophilic nature. Phylogenetic analysis shows that Penicillium is polyphyletic. The genus is re-defined and a monophyletic genus for both anamorphs and teleomorphs is created (Penicillium sensu stricto). The genera Thysanophora, Eupenicillium, Chromocleista, Hemicarpenteles and Torulomyces belong in Penicilliums. str. and new combinations for the species belonging to these genera are proposed. Analysis of Penicillium below genus rank revealed the presence of 25 clades. A new classification system including both anamorph and teleomorph species is proposed and these 25 clades are treated here as sections. An overview of species belonging to each section is presented.New sections, all in Penicillium: sect. Sclerotiora Houbraken & Samson, sect. Charlesia Houbraken & Samson, sect. Thysanophora Houbraken & Samson,sect. Ochrosalmonea Houbraken & Samson, sect. Cinnamopurpurea Houbraken & Samson, Fracta Houbraken & Samson, sect. Stolkia Houbraken & Samson, sect. Gracilenta Houbraken & Samson, sect. Citrina Houbraken & Samson, sect. Turbata Houbraken & Samson, sect. Paradoxa Houbraken & Samson, sect. Canescentia Houbraken & Samson. New combinations:Penicillium asymmetricum (Subramanian & Sudha) Houbraken & Samson, P. bovifimosum (Tuthill & Frisvad) Houbraken & Samson, P. glaucoalbidum (Desmazières) Houbraken & Samson, P. laeve (K. Ando & Manoch) Houbraken & Samson, P. longisporum (Kendrick) Houbraken & Samson, P. malachiteum (Yaguchi & Udagawa) Houbraken & Samson, P. ovatum (K. Ando & Nawawi) Houbraken & Samson, P. parviverrucosum (K. Ando & Pitt) Houbraken & Samson, P. saturniforme (Wang & Zhuang) Houbraken & Samson, P. taiwanense (Matsushima) Houbraken & Samson. New names:Penicillium coniferophilum Houbraken & Samson, P. hennebertii Houbraken & Samson, P. melanostipe Houbraken & Samson, P. porphyreum Houbraken & Samson.
Classification of Aspergillus, Penicillium, Talaromyces and related genera (Eurotiales): an overview of families, genera, subgenera, sections, series and species
DOI:10.1016/j.simyco.2020.05.002 URL [本文引用: 2]
Three new species of Talaromyces sect. Talaromyces discovered from soil in China
DOI:10.1038/s41598-018-23370-x URL [本文引用: 1]
International code of nomenclature for algae, fungi, and plants
The Talaromyces pinophilus species complex
DOI:S1878-6146(18)30307-6
PMID:31542192
[本文引用: 1]

A sample of isolates from Talaromyces pinophilus (55 isolates) and closely related species (76 isolates) was sequenced at four loci, the data were analyzed using maximum likelihood analysis and the GCPSR. The isolates were subjected to growth studies on the recommended media for description of Talaromyces species. On the basis of the combined data, five new species were segregated out of T. pinophilus and placed in newly described species. The T. pinophilus species complex contains ten species. The three other new species, Talaromyces argentinensis, T. californicus and T. louisianensis were not a part of the T. pinophilus species complex but occurred in Talaromyces sect. Talaromyces. T. argentinensis produces a teleomorphic state and is phylogenetically and morphologically distinct from other Talaromyces species.Published by Elsevier Ltd.
A manual of the penicillia
Color standards and color nomenclature
MRBAYES 3.2: Efficient Bayesian phylogenetic inference and model selection across a large model space
DOI:10.1093/sysbio/sys029
PMID:22357727
[本文引用: 1]

Since its introduction in 2001, MrBayes has grown in popularity as a software package for Bayesian phylogenetic inference using Markov chain Monte Carlo (MCMC) methods. With this note, we announce the release of version 3.2, a major upgrade to the latest official release presented in 2003. The new version provides convergence diagnostics and allows multiple analyses to be run in parallel with convergence progress monitored on the fly. The introduction of new proposals and automatic optimization of tuning parameters has improved convergence for many problems. The new version also sports significantly faster likelihood calculations through streaming single-instruction-multiple-data extensions (SSE) and support of the BEAGLE library, allowing likelihood calculations to be delegated to graphics processing units (GPUs) on compatible hardware. Speedup factors range from around 2 with SSE code to more than 50 with BEAGLE for codon problems. Checkpointing across all models allows long runs to be completed even when an analysis is prematurely terminated. New models include relaxed clocks, dating, model averaging across time-reversible substitution models, and support for hard, negative, and partial (backbone) tree constraints. Inference of species trees from gene trees is supported by full incorporation of the Bayesian estimation of species trees (BEST) algorithms. Marginal model likelihoods for Bayes factor tests can be estimated accurately across the entire model space using the stepping stone method. The new version provides more output options than previously, including samples of ancestral states, site rates, site d(N)/d(S) rations, branch rates, and node dates. A wide range of statistics on tree parameters can also be output for visualization in FigTree and compatible software.
Food and indoor fungi. 2nd ed
Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus Biverticillium
DOI:10.3114/sim.2011.70.04
PMID:22308048
[本文引用: 2]

The taxonomic history of anamorphic species attributed to Penicillium subgenus Biverticillium is reviewed, along with evidence supporting their relationship with teleomorphic species classified in Talaromyces. To supplement previous conclusions based on ITS, SSU and/or LSU sequencing that Talaromyces and subgenus Biverticillium comprise a monophyletic group that is distinct from Penicillium at the generic level, the phylogenetic relationships of these two groups with other genera of Trichocomaceae was further studied by sequencing a part of the RPB1 (RNA polymerase II largest subunit) gene. Talaromyces species and most species of Penicillium subgenus Biverticilliumsensu Pitt reside in a monophyletic clade distant from species of other subgenera of Penicillium. For detailed phylogenetic analysis of species relationships, the ITS region (incl. 5.8S nrDNA) was sequenced for the available type strains and/or representative isolates of Talaromyces and related biverticillate anamorphic species. Extrolite profiles were compiled for all type strains and many supplementary cultures. All evidence supports our conclusions that Penicillium subgenus Biverticillium is distinct from other subgenera in Penicillium and should be taxonomically unified with the Talaromyces species that reside in the same clade. Following the concepts of nomenclatural priority and single name nomenclature, we transfer all accepted species of Penicillium subgenus Biverticillium to Talaromyces. A holomorphic generic diagnosis for the expanded concept of Talaromyces, including teleomorph and anamorph characters, is provided. A list of accepted Talaromyces names and newly combined Penicillium names is given. Species of biotechnological and medical importance, such as P. funiculosum and P. marneffei, are now combined in Talaromyces. Excluded species and taxa that need further taxonomic study are discussed. An appendix lists other generic names, usually considered synonyms of Penicillium sensu lato that were considered prior to our adoption of the name Talaromyces.Taxonomic novelties:New species - Talaromyces apiculatus Samson, Yilmaz & Frisvad, sp. nov. New combinationsand names - Talaromyces aculeatus (Raper & Fennell) Samson, Yilmaz, Frisvad & Seifert, T. albobiverticillius (H.-M. Hsieh, Y.-M. Ju & S.-Y. Hsieh) Samson, Yilmaz, Frisvad & Seifert, T. allahabadensis (B.S. Mehrotra & D. Kumar) Samson, Yilmaz & Frisvad, T. aurantiacus (J.H. Mill., Giddens & A.A. Foster) Samson, Yilmaz, & Frisvad, T. boninensis (Yaguchi & Udagawa) Samson, Yilmaz, & Frisvad, T. brunneus (Udagawa) Samson, Yilmaz & Frisvad, T. calidicanius (J.L. Chen) Samson, Yilmaz & Frisvad, T. cecidicola (Seifert, Hoekstra & Frisvad) Samson, Yilmaz, Frisvad & Seifert, T. coalescens (Quintan.) Samson, Yilmaz & Frisvad, T. dendriticus (Pitt) Samson, Yilmaz, Frisvad & Seifert, T. diversus (Raper & Fennell) Samson, Yilmaz & Frisvad, T. duclauxii (Delacr.) Samson, Yilmaz, Frisvad & Seifert, T. echinosporus (Nehira) Samson, Yilmaz & Frisvad, comb. nov. T. erythromellis (A.D. Hocking) Samson, Yilmaz, Frisvad & Seifert, T. funiculosus (Thom) Samson, Yilmaz, Frisvad & Seifert, T. islandicus (Sopp) Samson, Yilmaz, Frisvad & Seifert, T. loliensis (Pitt) Samson, Yilmaz & Frisvad, T. marneffei (Segretain, Capponi & Sureau) Samson, Yilmaz, Frisvad & Seifert, T. minioluteus (Dierckx) Samson, Yilmaz, Frisvad & Seifert, T. palmae (Samson, Stolk & Frisvad) Samson, Yilmaz, Frisvad & Seifert, T. panamensis (Samson, Stolk & Frisvad) Samson, Yilmaz, Frisvad & Seifert, T. paucisporus (Yaguchi, Someya & Udagawa) Samson & Houbraken T. phialosporus (Udagawa) Samson, Yilmaz & Frisvad, T. piceus (Raper & Fennell) Samson, Yilmaz, Frisvad & Seifert, T. pinophilus (Hedgcock) Samson, Yilmaz, Frisvad & Seifert, T. pittii (Quintan.) Samson, Yilmaz, Frisvad & Seifert, T. primulinus (Pitt) Samson, Yilmaz & Frisvad, T. proteolyticus (Kamyschko) Samson, Yilmaz & Frisvad, T. pseudostromaticus (Hodges, G.M. Warner, Rogerson) Samson, Yilmaz, Frisvad & Seifert, T. purpurogenus (Stoll) Samson, Yilmaz, Frisvad & Seifert, T. rademirici (Quintan.) Samson, Yilmaz & Frisvad, T. radicus (A.D. Hocking & Whitelaw) Samson, Yilmaz, Frisvad & Seifert, T. ramulosus (Visagie & K. Jacobs) Samson, Yilmaz, Frisvad & Seifert, T. rubicundus (J.H. Mill., Giddens & A.A. Foster) Samson, Yilmaz, Frisvad & Seifert, T. rugulosus (Thom) Samson, Yilmaz, Frisvad & Seifert, T. sabulosus (Pitt & A.D. Hocking) Samson, Yilmaz & Frisvad, T. siamensis (Manoch & C. Ramírez) Samson, Yilmaz & Frisvad, T. sublevisporus (Yaguchi & Udagawa) Samson, Yilmaz & Frisvad, T. variabilis (Sopp) Samson, Yilmaz, Frisvad & Seifert, T. varians (G. Sm.) Samson, Yilmaz & Frisvad, T. verruculosus (Peyronel) Samson, Yilmaz, Frisvad & Seifert, T. viridulus Samson, Yilmaz & Frisvad.
Multilocus phylogenetic analysis of Talaromyces species isolated from cucurbit plants in China and description of two new species, T. cucurbitiradicus and T. endophyticus
DOI:10.1080/00275514.2018.1432221 URL [本文引用: 1]
New section and species in Talaromyces
DOI:10.3897/mycokeys.68.52092 URL
The importance of Talaromyces and its taxonomy
Penicillium and related teleomorphs from Taiwan
Five new Talaromyces species with ampulliform-like phialides and globose rough walled conidia resembling T. verruculosus
DOI:10.1016/j.myc.2015.02.005 URL [本文引用: 2]
Four new records of Aspergillus section Usti from Shandong Province, China
DOI:10.5248/120.373 URL [本文引用: 1]
Two new records of Talaromyces section Islandici species from China
Designing primer sets for amplification of partial calmodulin genes from penicillia
Phylogenetic analyses of penicillia based on partial calmodulin gene sequences
DOI:10.1016/j.biosystems.2006.04.008 URL [本文引用: 1]
Talaromyces neofusisporus and T. qii, two new species of section Talaromyces isolated from plant leaves in Tibet, China
DOI:10.1038/srep18622 URL [本文引用: 1]
Talaromyces heiheensis and T. mangshanicus, two new species from China
DOI:10.1007/s11557-016-1251-3 URL [本文引用: 1]
A new species of Talaromyces, Trichocomaceae, from the Xisha Islands, Hainan, China
DOI:10.11646/phytotaxa.267.3 URL [本文引用: 1]
Delimitation and characterisation of Talaromyces purpurogenus and related species
DOI:10.3767/003158512X659500 URL [本文引用: 1]
Polyphasic taxonomy of the genus Talaromyces
DOI:10.1016/j.simyco.2014.08.001 URL [本文引用: 1]