杜鹃兰Cremastra appendiculata为兰科杜鹃兰属的多年生药用草本植物,有多种抗肿瘤活性成分(Liu et al. 2015;Liu et al. 2016),由于杜鹃兰自身繁殖困难和人为过度采挖使其野生资源濒临枯竭(郭顺星和徐锦堂 1990;王汪中等 2017)。太白山自然保护区地处秦岭山区中段,孕育了丰富的兰科植物资源(杨平厚等 2004),黄永会等(2007)在杜鹃兰根系中观察到典型的兰科植物菌根结构,并发现部分杜鹃兰菌根真菌能够促进杜鹃兰种子萌发(Yagame et al. 2013)和外植体成苗(Zhang et al. 2006)。兰科植物的生长发育依赖于菌根真菌提供营养(Lee et al. 2015;高越等 2019),且共生关系几乎伴随其整个生活史(Dearnaley et al. 2016)。Yagame et al.(2013)从日本神奈川县杜鹃兰根部分离到多株鬼伞属Coprinellus真菌,朱国胜(2009)从贵州施秉县杜鹃兰根部分离到的念珠菌根菌属Moniliopsis solani为杜鹃兰发育早期的优势菌根真菌,表明杜鹃兰根部优势内生真菌种类在不同地域存在差异。
自然状态下兰科植物与菌根真菌的共生会受到土壤类型和营养状况(盖雪鸽等 2014)、植被类型和海拔高度(徐玲玲等 2019;Lin et al. 2020)等多种因素的影响,土壤中菌根真菌的分布也会影响兰科植物的分布(Shefferson et al. 2005)。海拔梯度包含温度、湿度、土壤理化性质等各种环境因子,比纬度梯度变化更快,能够直接或间接影响植物根际真菌群落多样性(厉桂香和马克明 2018)。研究海拔梯度对真菌群落多样性的影响对研究生物多样性分布格局及其驱动因素具有重要意义。本研究选择同一栖息地下不同海拔杜鹃兰作为研究对象,对杜鹃兰根部内生真菌进行分离鉴定,分析太白山自然保护区蒿坪站野生杜鹃兰根部内生真菌的多样性及根际土理化性质,探究在同一生境中,不同海拔杜鹃兰根部内生真菌多样性和群落结构的变化规律,为我国野生兰科植物的培养和保护提供理论依据。
1 材料与方法
1.1 样地设置
杜鹃兰根系采集于陕西太白山国家自然保护区蒿坪站。地处秦岭山脉中段,属典型的内陆季风气候,海拔1 060-3 767m,年均气温12.9℃,年均降水量580mm(杨平厚等 2004)。2017年11月采取样地详查的方法调查野生杜鹃兰分布,根据其自然分布情况,于海拔1 177m、1 409m和1 590m处分别设置3个样地,每个样地设置10m×10m的样方,每个样方随机选取5株杜鹃兰进行采样。
图1
图1
野生杜鹃兰及根内菌丝团形态
A:自然状态下野生杜鹃兰;B,C:野生杜鹃兰根内菌丝团. P表示菌丝团,CO表示皮层,EX表示外皮层,PE表示中柱鞘,RH表示根毛,VE表示根被
Fig. 1
Wild Cremastra appendiculata and the morphology of pelotons in the roots.
A: The nature state of C. appendiculata; B and C: The morphology of pelotons in the roots of C. appendiculata observed under microscope. P=Pelotons, CO=Cortex, EX=External cortex, PE=Pericycle, RH=Root hair, VE=Velamen.
1.2 根际土理化性质测定
根际土测定鲜土含水量(烘干称重法);剩余土样自然风干,过0.15mm筛后采用鲍士旦(2000)的方法测定速效磷(碳酸氢钠浸提-钼锑抗比色法)、速效钾(乙酸铵浸提-火焰光度法)、硝态氮(氯化钾浸提-流动分析法)、铵态氮(氯化钾浸提-流动分析法)和有机质含量(外加热-重铬酸钾容量法)。
1.3 杜鹃兰根部内生真菌分离
真菌分离培养基为马铃薯燕麦琼脂培养基(potato oats agar,POA)(孙晓颖等 2015)、PDA和Martin琼脂培养基(侯天文等 2010)。采用组织分离法从菌丝团和根组织中分离根部内生真菌。将根系用75%乙醇和1%次氯酸钠表面消毒后,切成长2cm根段,均匀分成2组。一组切成0.5mm的薄片,平铺于3种培养基上,每皿10个(侯天文等 2010)。另一组用灭菌解剖刀将根皮层细胞刮下,置于无菌研钵中,加1mL无菌水碾碎制成菌丝团悬浮液,取8μL至3种培养基块(9mm)中央,每皿10块(孙晓颖等 2015)。28℃恒温暗培养,待有菌丝长出,挑取菌丝至PDA培养基上纯化后,转接到PDA斜面培养基上,4℃冰箱保存。
1.4 真菌鉴定
采用真菌DNA提取试剂盒(Fungal DNA Kit,Omega,America)提取DNA,以ITS1-F(5’-CTTGGTCATTTAGAGGAAGTAA-3’)(Gardes & Bruns 1993)和ITS4(5’-TCCTCCGCTTATTGA TATGC-3’)(White et al. 1990)为引物,扩增真菌转录间隔区(internal transcribed spacer,ITS)序列进行分子鉴定。采用40μL体系:模板2μL、2×Premix Taq 20μL(TaKaRa,Japan)、上游引物ITS1-F 1μL(10µmol/L)、下游引物ITS4 1μL(10µmol/L)、ddH2O 16μL。PCR条件:94℃预变性3min;35个循环,94℃变性30s,55℃退火30s,72℃延伸1min;最终72℃延伸5min(Seifert 2009)。经1%琼脂糖凝胶电泳(120V,30min)检测,PCR产物为单一条带,送至奥科鼎盛生物科技有限公司(北京)测序,测序结果上传至GenBank数据库(
1.5 杜鹃兰根部内生真菌多样性分析
1.5.1 DNA提取及巢式PCR:新鲜根经液氮研磨后,用DNA提取试剂盒提取DNA(Plant Genomic DNA kit,Tiangen,China),经Nanodrop和1%琼脂糖凝胶电泳(120V,30min)测定DNA浓度和质量(侯天文等 2010),参照Yu et al.(2013)的方法进行巢式PCR。第一轮PCR为20μL体系:模板1μL、2×Premix Taq 10μL、上游引物ITS1-F 0.5μL(10µmol/L)、下游引物ITS4 0.5μL(10µmol/L)、ddH2O 8μL。PCR条件:94℃预变性3min;35个循环,94℃变性1min,56℃退火1min,72℃延伸1min;最终72℃延伸10min。第二轮PCR为40μL体系:模板(第一轮PCR产物稀释100倍)2μL、2×Premix Taq 20μL、上游引物ITS1-F-GC(5’-CGCCCGCCGCGCGCGGCGG GCGGGGCGGGGGCACGGGGGGCTTGGTCATTTAGAGGAAGTAA-3’,下划线处为GC发夹结构)1μL(10µmol/L)、下游引物ITS2(5’-GCTGCGT TCTTCATCGATGC-3’)1μL(10µmol/L)、ddH2O 16μL(Gardes & Bruns 1993)。PCR条件:94℃预变性5min;35个循环,94℃变性45s,56℃退火45s,72℃延伸45s;最终72℃延伸5min。经1%琼脂糖凝胶电泳检测,第二轮PCR产物为单一条带,进行变性梯度凝胶电泳。
1.5.2 变性梯度凝胶电泳(denaturing gradient gel electrophoresis,DGGE):参照Strathdee & Free(2013)的方法配制变性梯度胶,浓度为30%-40%,从胶的上方向下方递增。取巢式PCR第二轮产物40µL加入加样孔中,电泳缓冲液为1×TAE,60℃,70V预电泳30min,120V电泳5h。电泳完毕,将胶块放入含核酸染液(Gel Stain,TransGen,China)的1×TAE中染色30min,在凝胶自动成像仪上拍照、保存,用于杜鹃兰根部内生真菌多样性分析。将DGGE凝胶各泳道最亮的条带切下,置入100μL无菌水中4℃浸泡24h作为模板,以巢式PCR第二轮程序不含GC发夹结构的引物ITS1-F和ITS2扩增ITS区用于优势菌鉴定(Yu et al. 2013),经1%琼脂糖凝胶电泳检测后,单一条带送至奥科鼎盛生物科技有限公司(北京)测序,测序结果已上传至GenBank数据库,GenBank登录号为MT929301- MT929309。
1.6 数据处理
使用Microsoft Office Excel(Version 2016)统计数据,SPSS(Version 19.0)进行单因素方差分析(Duncan’s test)和相关性分析(Pearson’s correlation coefficient),MetaboAnalyst(Version 4.0)进行主成分分析(Chong et al. 2019)。DGGE图谱通过Quantity One(Version 4.41)软件进行数字化处理,将条带的亮度转换为峰密度值分析真菌多样性,依据公式将得到的峰密度值转为辛普森指数(D)、均匀度指数(Eh)和香侬维纳指数(H),丰富度指数(S)以各泳道出现的条带数表示。S为DGGE图谱中的不同条带数的总和,N为各泳道中检测到所有条带的峰密度的总和,Ni为第i条泳道的峰密度值(Yu et al. 2013)。
$\begin{align} & H=-\sum\limits_{i=1}^{s}{PilnPi=-\sum\limits_{i=1}^{s}{(Ni/N)ln(Ni/N)}} \\ & {{E}_{h}}=H/{{H}_{max}}=H/lnS \\ & D=\sum\limits_{i=1}^{s}{{{(Ni/N)}^{2}}} \\ \end{align}$
2 结果与分析
2.1 杜鹃兰根部内生真菌鉴定及系统发育分析
采用组织分离法从根组织和菌丝团在3种培养基上分离到79株真菌,通过比对真菌ITS序列鉴定为19种(表1)。19种真菌E值均小于10-5,表明序列比对的可靠性高。除M251、M342、O322B和TP331B以外,其余15种真菌ITS序列一致性均达到98%以上,认为是同种真菌。结合系统发育分析,M251为角担菌科Ceratobasidiaceae真菌,M342为刺盘孢菌属Colletotrichum真菌,O322B为毛壳菌属Chaetomium真菌,TP331B为木霉属Trichoderma真菌。系统发育树中,19种真菌ITS序列与GenBank数据库相似度最高序列能够聚在一起,且隶属于同一目的真菌聚在一起,与分子鉴定结果一致(图2)。
表1 根部内生真菌鉴定结果
Table 1
| 菌株号 Strain No. | 登录号 Accession No. | 分类地位 Taxonomic affiliation | GenBank最相似序列 Closest match in GenBank | 序列一致性 Sequence identity (%) | E值 E-value | 分离 频率 IF (%) | 分离方式 Isolated method |
|---|---|---|---|---|---|---|---|
| M111 | MT920565 | 小丛壳科 Glomerellaceae | 麦冬刺盘孢 Colletotrichum liriopes isolate HZ-1 (MK644098.1) | 98.80 | 0 | 8 | 根组织 Roots 菌丝团 Pelotons |
| M112 | MT920566 | 小双腔菌科 Didymellaceae | Paraboeremia putaminum strain CBS 538.66 (MH858878.1) | 99.62 | 0 | 3 | 根组织 Roots |
| M251 | MT920567 | 角担菌科 Ceratobasidiaceae | Ceratobasidiaceae sp. CBS 510.93 (KF267009.1) | 82.39 | 2E-32 | 1 | 根组织 Roots |
| M311 | MT920568 | 炭角菌科 Xylariaceae | 多节孢属 Nodulisporium sp. JP60-3 (GQ906948.1) | 98.56 | 0 | 14 | 根组织 Roots |
| M321Y | MT920569 | 小丛壳科 Glomerellaceae | 大豆刺盘孢 Colletotrichum truncatum strain BBA 70523 (AJ301937.1) | 98.96 | 0 | 4 | 根组织 Roots |
| M322 | MT920570 | 丛赤壳科 Nectriaceae | 锈腐土赤壳 Ilyonectria destructans isolate UASWS1480 (KT722597.1) | 100.00 | 0 | 3 | 根组织 Roots |
| M342 | MT920571 | 小丛壳科 Glomerellaceae | Colletotrichum rhombiforme isolate RP257_2 (KX067811.1) | 74.29 | 2E-17 | 1 | 根组织 Roots |
| M353MZ | MT920572 | 炭角菌科 Xylariaceae | Nemania serpens voucher KoLRI_ EL006190 (MN844433.1) | 98.99 | 0 | 5 | 根组织 Roots |
| O112 | MT920573 | 麦角菌科 Clavicipitaceae | 厚垣轮枝孢菌 Metacordyceps chlamydosporia isolate KYK00228 (AB378549.1) | 99.67 | 0 | 6 | 根组织 Roots |
| O311 | MT920574 | 团壳菌科 Boliniaceae | Camaropella pugillus strain CBS 128346 (MH864891.1) | 98.69 | 0 | 6 | 根组织 Roots |
| O322B | MT920575 | 毛壳菌科 Chaetomiaceae | 球毛壳菌 Chaetomium globosum isolate FMB-HP03 (MF716852.1) | 93.57 | 0 | 3 | 根组织 Roots |
| P142 | MT920576 | 丛赤壳科 Nectriaceae | Dactylonectria macrodidyma strain GFR05 (MT447510.1) | 99.09 | 0 | 4 | 根组织 Roots |
| P231 | MT920577 | 毛孢壳科 Coniochaetaceae | Coniochaeta mutabilis strain KRP51-8 (HM036599.1) | 99.63 | 0 | 3 | 根组织 Roots |
| P321 | MT920578 | 小丛壳科 Glomerellaceae | 高代花刺盘孢 Colletotrichum godetiae isolate A49 (LT717068.1) | 99.15 | 0 | 1 | 根组织 Roots |
| P353 | MT920579 | 线孢虫草科 Ophiocordycipitaceae | 白色弯颈霉 Tolypocladium album strain CBS 830.73 (MH860811.1) | 98.79 | 0 | 3 | 根组织 Roots |
| TM121 | MT920580 | 肉座菌科 Hypocreaceae | 多孢木霉 Trichoderma polysporum isolate TR3.2 (KX343125.1) | 98.87 | 0 | 23 | 根组织 Roots 菌丝团 Pelotons |
| TP131 | MT920581 | 毛孢壳科 Coniochaetaceae | 木生锥毛壳 Coniochaeta ligniaria strain B121 (KX090317.1) | 99.26 | 0 | 1 | 菌丝团 Pelotons |
| TP321H | MT920582 | 隔孢腔菌科 Pleosporaceae | 交链链格孢 Alternaria alternata isolate RdKnA-5 (MF167641.1) | 99.48 | 0 | 5 | 根组织 Roots 菌丝团 Pelotons |
| TP331B | MT920583 | 肉座菌科 Hypocreaceae | 装絮木霉 Trichoderma tomentosum strain DAOM 195050 (AY605717.1) | 95.35 | 0 | 8 | 根组织 Roots 菌丝团 Pelotons |
图2
图2
基于ITS序列构建的内生真菌系统发育树
Fig. 2
Phylogenetic tree based on ITS sequences of endophytic fungi in roots of Cremastra appendiculata.
19种真菌均属于子囊菌门Ascomycota,隶属于2纲8目12科(表1)。其中座囊菌纲Dothideomycetes 1目2科2种,小双腔菌科Didymellaceae和隔孢腔菌科Pleosporaceae各1种;粪壳菌纲Sordariomycetes 7目10科17种,最多的为小丛壳科Glomerellaceae 4种,肉座菌科Hypocreaceae、丛赤壳科Nectriaceae、毛孢壳科Coniochaetaceae和炭角菌科Xylariaceae各2种,线孢虫草菌科Ophiocordycipitaceae、麦角菌科Clavicipitaceae、角担菌科、团壳菌科Boliniaceae和毛壳菌科Chaetomiaceae各1种。其中,麦冬刺盘孢Colletotrichum liriopes、多孢木霉Trichoderma polysporum、交链链格孢Alternaria alternata和木霉属Trichoderma sp.从根组织和菌丝团中均能分离得到。肉座菌科、炭角菌科和小丛壳科真菌分离频率较高,分别为31%、19%和14%,其中肉座菌科的多孢木霉Trichoderma polysporum和炭角菌科的多节孢属真菌Nodulisporium sp.分离频率分别为23%和14%。
2.2 不同海拔野生杜鹃兰根部内生真菌多样性
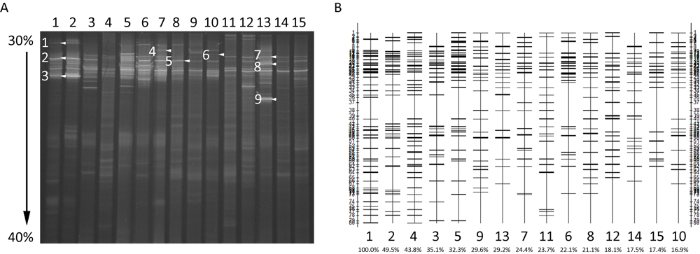
图3
图3
根部内生真菌DGGE图谱及泳道分析
A:泳道顶部数字为样品号,1-5、6-10和11-15分别为1 177m、1 409m和1 590m海拔样地;箭头所指条带为优势菌条带. B:泳道底部数字为对应图A中样品编号,编号下为与1泳道的相似系数
Fig. 3
DGGE profiles and lane analysis of endophytic fungi in roots of Cremastra appendiculata.
The number at the top of lane in A represented the sample number. 1-5, 6-10 and 11-15 sampled in altitudes of 1 177m, 1 409m and 1 590m respectively. The band with arrow represented the dominant fungi. The number at the bottom of lane in B was the sample number in Fig. A. The number under sample number was the similarity coefficient with lane 1.
各样地优势菌序列与GenBank数据库比对信息见表2。条带5的序列一致性为96.23%,为柔膜菌科Helotiaceae真菌,条带6的序列一致性为97.78%,为链格孢属Alternaria真菌,条带8的序列一致性为97.09%,为锤舌菌纲Leotiomycetes真菌,其余条带序列一致性大于98%,可确认为同一种。E值均小于10-5,表明序列比对的可靠性高。拟锁瑚菌属真菌Clavulinopsis sp.(HQ021970.1)和锈腐土赤壳I. destructans(MN540280.1)在3个海拔下均为优势菌。1 177m海拔处特有优势菌为乳菇属的Lactarius gymnocarpoides(LK392600.1)和四枝孢属的Tetracladium maxilliforme(MK353128.1),1 409m海拔处特有优势菌为丝核菌属真菌Rhizoctonia sp.(DQ061931.1)、膜盘菌属真菌Hymenoscyphus sp.(AB456655.1)和链格孢属真菌Alternaria sp.(MT594775.1),1 590m海拔处特有优势菌为锤舌菌纲真菌Leotiomycetes sp.(FJ553339.1)和哈茨木霉T. harzianum(MT594389.1),说明不同海拔下杜鹃兰与内生真菌的共生关系存在差异。
表2 DGGE分析优势菌鉴定结果
Table 2
| 条带号 Band No. | 海拔 Altitude (m) | 登录号 Accession No. | 分类地位 Taxonomic affiliation | GenBank最相似序列 Closest match in GenBank | 序列一致性 Sequence identity (%) | E值 E-value |
|---|---|---|---|---|---|---|
| 1 | 1 177 | MT929301 | 红菇科 Russulaceae | Lactarius gymnocarpoides strain MD318 (LK392600.1) | 100 | 5E-10 |
| 2 | 1 177 | MT929302 | 子囊菌类 Ascomycota incertae sedis | Tetracladium maxilliforme strain DSM105583 (MK353128.1) | 100 | 4E-141 |
| 3 | 1 177, 1 409, 1 590 | MT929303 | 珊瑚菌科 Clavariaceae | Uncultured Clavulinopsis sp. clone 4Bart1300S (HQ021970.1) | 100 | 6E-11 |
| 4 | 1 409 | MT929304 | 伞菌亚门 Agaricomycotina | Uncultured Rhizoctonia sp. isolate IT1B-10r (DQ061931.1) | 100 | 5E-13 |
| 5 | 1 409 | MT929305 | 柔膜菌科 Helotiaceae | Uncultured Hymenoscyphus sp. clone ITS-09 (AB456655.1) | 96.23 | 1E-13 |
| 6 | 1 409 | MT929306 | 隔孢腔菌科 Pleosporaceae | 交链链格孢Alternaria alternata strain OTU1257 (MT594775.1) | 97.78 | 9E-11 |
| 7 | 1 177, 1 409, 1 590 | MT929307 | 丛赤壳科 Nectriaceae | 锈腐土赤壳 Ilyonectria destructans (MN540280.1) | 98.80 | 4E-121 |
| 8 | 1 590 | MT929308 | 锤舌菌纲 Leotiomycetes | Uncultured ectomycorrhiza (Leotiomycetes) clone LTSP_ EUKA_ P3A23 (FJ553339.1) | 97.09 | 4E-126 |
| 9 | 1 590 | MT929309 | 肉座菌科 Hypocreaceae | 哈茨木霉Trichoderma harzianum strain 104-D12 (MT594389.1) | 100 | 3E-15 |
注:条带号对应
Note: The band No. corresponds to the band No. of dominant fungi in
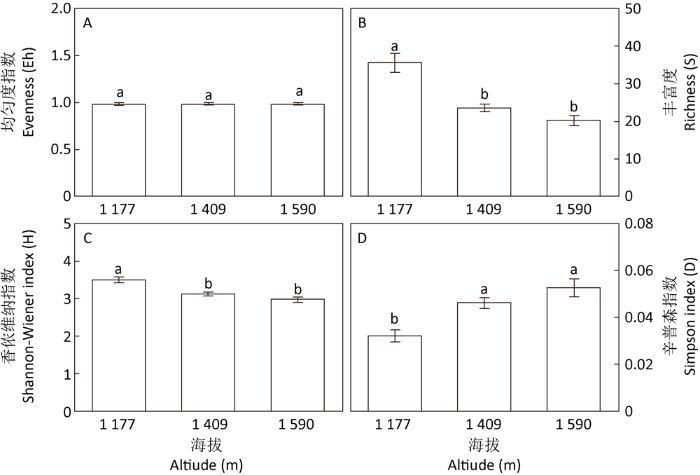
图4
图4
不同海拔样地真菌群落多样性指数
柱子表示均值±标准误,柱子上不同小写字母表示差异显著(Duncan检验,P<0.05,n=5)
Fig. 4
The diversity indices of fungal community in different sample sites. Bars indicate mean±SE.
The different lowercase letters on the bars mean that there is significant difference by Duncan’s test (P<0.05, n=5).
海拔变化对太白山杜鹃兰根部内生真菌群落丰富度、香侬维纳指数和辛普森指数影响较大,海拔越高内生真菌越少,群落分布越不均匀,优势真菌越突出。
2.3 不同海拔下杜鹃兰根部内生真菌多样性与土壤理化性质的关系
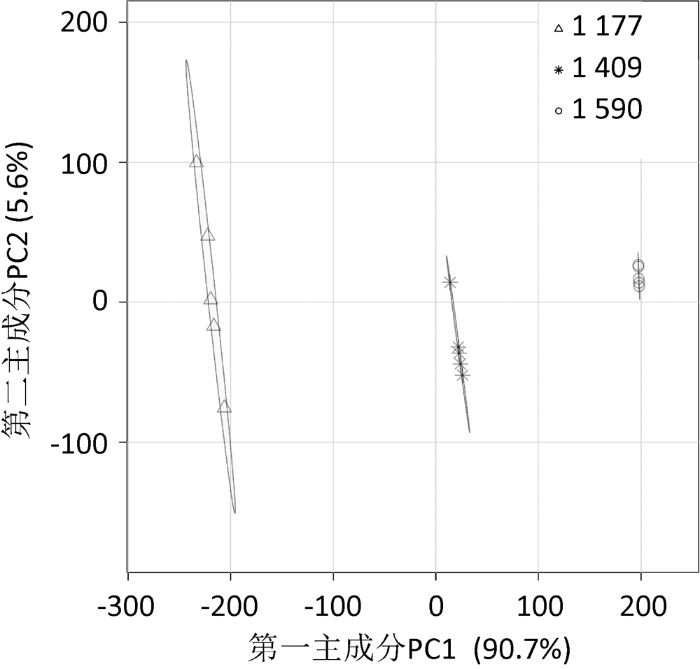
主成分分析(principal component analyses,PCA)海拔与杜鹃兰根部内生真菌多样性和样地根际土理化性质的关系(图5),第一主成分(principal component 1,PC1)和第二主成分(principal component 2,PC2)的总解释量为96.3%,PC1和PC2将3个海拔样地分开,表明不同海拔野生杜鹃兰根部内生真菌多样性和根际土理化性质存在显著差异。
图5
图5
不同海拔样地真菌群落多样性与根际土理化性质的主成分分析
1 177、1 409和1 590分别表示1 177、1 409和1 590m海拔的3个样地,n=5
Fig. 5
Principal component analysis plot of the fungal community diversity indices and rhizosphere soil factors in different altitude sample sites.
1 177, 1 409 and 1 590 respectively represented 3 sample sites at altitudes of 1 177, 1 409 and 1 590m, n=5.
表3 不同海拔杜鹃兰根际土土壤理化性质
Table 3
| 根际土理化性质 Rhizosphere soil factors | 样地海拔Sample site altitudes (m) | ||
|---|---|---|---|
| 1 177 | 1 409 | 1 590 | |
| 有机质 Organic matter (g/kg) | 119.50±25.90a | 68.46±17.08a | 72.14±3.72a |
| 含水量 Water content | 0.33±0.03a | 0.38±0.02a | 0.36±0.01a |
| 硝态氮 Nitrate N (mg/kg) | 67.27±25.01a | 13.28±1.29b | 36.13±2.10ab |
| 铵态氮 Ammonium N (mg/kg) | 43.77±11.58a | 29.94±1.41a | 48.07±6.29a |
| 速效磷 Available P (mg/kg) | 50.15±1.17a | 30.06±5.29b | 34.61±6.84b |
| 速效钾 Available K (mg/kg) | 72.91±4.66a | 80.99±3.14a | 77.20±1.39a |
注:表中数据为均值±标准误(SE),同行不同字母代表差异显著(Duncan检验,P<0.05,n=5)
Note: The data in the table is the mean±standard error (SE), n=5. Different letters within a row indicate the values are significantly different at the level of P<0.05 by Duncan’s test.
表4 根际土理化性质与真菌多样性之间的相关性分析
Table 4
| 根际土理化性质 Rhizosphere soil factors | 相关系数Pearson’s correlation index | |||
|---|---|---|---|---|
| 香侬维纳指数 Shannon-Wiener index | 丰富度 Richness | 均匀度指数 Evenness | 辛普森指数 Simpson index | |
| 海拔Altitude | -0.858** | -0.868** | 0.484 | 0.821** |
| 含水量Water content | -0.049 | -0.054 | 0.293 | 0.055 |
| 硝态氮Nitrate N | 0.607* | 0.652** | 0.087 | -0.534* |
| 铵态氮Ammonium N | 0.266 | 0.280 | 0.437 | -0.235 |
| 速效磷Available P | 0.513 | 0.519* | -0.067 | -0.497 |
| 速效钾Available K | -0.432 | -0.467 | 0.019 | 0.373 |
| 有机质Organic matter | 0.323 | 0.368 | -0.365 | -0.267 |
注:*表示相关性达显著水平(P<0.05),**表示相关性达极显著水平(P<0.01),n=5
Note: * represents statistical significant at 0.05 level (P<0.05), ** represents statistical significant at 0.01 level (P<0.01), n=5.
3 讨论
对兰科植物菌根真菌角担菌科(López- Chávez et al. 2016)和胶膜菌科Tulasnellaceae的研究较多(盖雪鸽等 2014)。本研究以组织分离法从太白山蒿坪站3个海拔杜鹃兰根部分离到的内生真菌19种,均为子囊菌门,其中小丛壳科、肉座菌科、丛赤壳科、角担菌科、毛壳菌科、小双腔菌科和孢腔菌科的12种真菌属于兰科植物菌根真菌(郭顺星 2016)。DGGE检测到的真菌共80种,在组织分离法的基础上新检测到担子菌门红菇科、珊瑚菌科和伞菌亚门的真菌3种,子囊菌门柔膜菌科的真菌1种,明显多于组织分离法检测到的真菌。DGGE直接以样本中DNA进行分析,不受培养方式的影响,能够更准确地反映植物内生真菌的多样性(Strathdee & Free 2013)。DGGE的缺点在于不能直接获得可培养菌株。组织分离得到的真菌,可进行共生培养等研究,对进一步了解兰科植物与菌根真菌共生关系和兰科植物的人工培育繁殖具有非常重要的意义。但组织分离法会受培养基成分、培养方法等因素的限制,且自然界中绝大多数微生物尚不能被目前的纯培养技术培养(Rappé & Giovannoni 2003)。DGGE检测的9种优势菌中,通过组织分离法仅得到锈腐土赤壳、交链链格孢以及哈茨木霉同属的木霉属真菌,其余真菌不能从组织分离中得到。因此,将组织分离法和DGGE结合起来能够更全面且准确地反映植物内生真菌的多样性。
在组织分离和DGGE中均检测到兰科菌根真菌锈腐土赤壳、交链链格孢和木霉属真菌,表明锈腐土赤壳、交链链格孢和木霉属真菌可能在太白山区杜鹃兰的生长发育过程中起到至关重要的作用。其中,在3个海拔样地均检测到锈腐土赤壳,表明太白山区杜鹃兰可能与锈腐土赤壳关系更紧密。吴慧凤等(2012)发现链格孢属真菌可以促进铁皮石斛Dendrobium catenatum原球茎发育和幼苗生长,但部分兰科菌根真菌在其他宿主植物上表现为病原菌,如交链链格孢和锈腐土赤壳(傅本重等 2012)。同种真菌在不同兰科植物的不同生长发育阶段有不同的作用(Favre-Godal et al. 2020),如木霉属真菌为兼性腐生真菌,能拮抗多种植物病原真菌、增强菌根真菌的作用(Yuan et al. 2016),促进春兰和大花蕙兰的杂交苗生长(黄磊等 2004)。太白山区蒿坪站杜鹃兰根部优势内生真菌与Yagame et al.(2013)从日本神奈川县杜鹃兰根部分离到的鬼伞属Coprinellus真菌和朱国胜(2009)从贵州施秉县杜鹃兰根部分离到的念珠菌根菌属Moniliopsis solani不同,进一步证实杜鹃兰根部优势内生真菌种类存在地理差异。
兰科植物菌根真菌群落多样性受海拔的影响。徐玲玲等(2019)对四川黄龙沟不同海拔的西藏杓兰菌根真菌群落多样性研究表明,海拔升高兰科菌根真菌多样性降低。本研究取样点位于杜鹃兰同一栖息地不同海拔处,海拔升高,杜鹃兰根部内生真菌越少,群落分布越不均匀,优势真菌越突出。海拔的变化通常伴随着土壤养分的变化(厉桂香和马克明 2018)。菌根真菌能够改变土壤中氮和磷的有效性,为植物提供氮和磷元素(Bell et al. 2020),尤其是兰科植物的生长发育对菌根真菌提供营养的依赖性更强(Lee et al. 2015),土壤养分也能调节兰科菌根真菌与植物的共生关系(邓文祥等 2019;Mujica et al. 2020)。太白山蒿坪站杜鹃兰同一栖息地不同海拔处,根际土有机质、含水量、速效钾和铵态氮等营养元素含量无明显差异,但硝态氮和速效磷含量在1 177m海拔显著高于1 409m和1 590m,随海拔升高杜鹃兰根部内生真菌群落丰富度和香侬维纳指数降低。Gebauer & Meyer(2003)通过稳定同位素分析表明兰科植物能够从菌根真菌中获得碳和氮,土壤速效氮和速效磷含量对菌根侵染率有较大的影响(盖雪鸽等 2014)。土壤氮和磷水平是驱动土壤微生物多样性和群落结构改变的重要因子,影响着微生物代谢(Leff et al. 2015)。因此,根际土速效磷和硝态氮含量不同,可能是造成太白山杜鹃兰根部内生真菌群落随海拔变化的原因。
本研究分离得到的兰科菌根真菌交链链格孢、锈腐土赤壳和木霉属真菌可能与杜鹃兰生长关系更密切,后续可以对它们之间的共生关系与营养元素氮和磷吸收的机制进行进一步研究。对同一栖息地不同海拔下兰科菌根真菌群落多样性进行调查研究,有利于发掘此地区杜鹃兰菌根真菌资源,对兰科植物的人工培育繁殖以及物种多样性保护都有重要意义。
参考文献
Diversity of root-associated culturable fungi of Cephalanthera rubra (Orchidaceae) in relation to soil characteristics
Diversity of endophytic fungi from Pteroceltis tatarinowii
Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis
Molecular characterization of fungal endophytic morphospecies isolated from stems and pods of Theobroma cacao
DOI:10.1111/ppa.2006.55.issue-6 URL [本文引用: 1]
Diversity of endophytic fungi associated with Bletilla striata roots
Orchids and their mycorrhizal fungi: an insufficiently explored relationship
DOI:10.1007/s00572-020-00934-2
PMID:31982950
[本文引用: 1]

Orchids are associated with diverse fungal taxa, including nonmycorrhizal endophytic fungi as well as mycorrhizal fungi. The orchid mycorrhizal (OM) symbiosis is an excellent model for investigating the biological interactions between plants and fungi due to their high dependency on these symbionts for growth and survival. To capture the complexity of OM interactions, significant genomic, numerous transcriptomic, and proteomic studies have been performed, unraveling partly the role of each partner. On the other hand, several papers studied the bioactive metabolites from each partner but rarely interpreted their significance in this symbiotic relationship. In this review, we focus from a biochemical viewpoint on the OM dynamics and its molecular interactions. The ecological functions of OM in plant development and stress resistance are described first, summarizing recent literature. Secondly, because only few studies have specifically looked on OM molecular interactions, the signaling pathways and compounds allowing the establishment/maintenance of mycorrhizal association involved in arbuscular mycorrhiza (AM) are discussed in parallel with OM. Based on mechanistic similarities between OM and AM, and recent findings on orchids' endophytes, a putative model representing the different molecular strategies that OM fungi might employ to establish this association is proposed. It is hypothesized here that (i) orchids would excrete plant molecule signals such as strigolactones and flavonoids but also other secondary metabolites; (ii) in response, OM fungi would secrete mycorrhizal factors (Myc factors) or similar compounds to activate the common symbiosis genes (CSGs); (iii) overcome the defense mechanism by evasion of the pathogen-associated molecular patterns (PAMPs)-triggered immunity and by secretion of effectors such as small inhibitor proteins; and (iv) finally, secrete phytohormones to help the colonization or disrupt the crosstalk of plant defense phytohormones. To challenge this putative model, targeted and untargeted metabolomics studies with special attention to each partner's contribution are finally encouraged and some technical approaches are proposed.
Alternaria alternata, India canna black leaf spot pathogen in Kunming
Ecological research of orchid mycorrhizae: a review
Fungal diversity and mechanisms of symbiotic germination of orchid seeds: a review
ITS primers with enhanced specificity for basidiomycetes application to the identification of mycorrhizae and rusts
DOI:10.1111/j.1365-294X.1993.tb00005.x URL [本文引用: 2]
15N and 13C natural abundance of autotrophic and myco-heterotrophic orchids provides insight into nitrogen and carbon gain from fungal association
DOI:10.1046/j.1469-8137.2003.00872.x URL [本文引用: 1]
Study on seeds vitro germination of orchid
The variations of mycorrhizal fungi diversity among different growing periods of the dominant orchids from two habitats in the Huanglong valley, Sichuan
Screening of genes related to early and late flowering in tree peony based on bulked segregant RNA sequencing and verification by quantitative real-time PCR
DOI:10.3390/molecules23010001 URL [本文引用: 1]
Preliminary studies on mycorrhizal fungi in promoting the growth of orchid seedings from tissue culture
Primary study on mycorrhizal microstructure of Cremastra appendiculata (D. Don) Makino
The importance of associations with saprotrophic non-Rhizoctonia fungi among fully mycoheterotrophic orchids is currently under-estimated: novel evidence from sub-tropical Asia
DOI:10.1093/aob/mcv085 URL [本文引用: 2]
Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe
Progress in the study of elevational patterns of soil microbial diversity
The effect of plant geographical location and developmental stage on root-associated microbiomes of Gymnadenia conopsea
DOI:10.3389/fmicb.2020.00001 URL [本文引用: 1]
Five new benzylphenanthrenes from Cremastra appendiculata
DOI:10.1016/j.fitote.2015.03.003 URL [本文引用: 1]
Two new phenanthrene glucosides from Cremastra appendiculata and their cytotoxic activities
Proteomic and morphometric study of the in vitro interaction between Oncidium sphacelatum Lindl. (Orchidaceae) and Thanatephorus sp. RG26 (Ceratobasidiaceae)
DOI:10.1007/s00572-015-0676-x
PMID:26732875
[本文引用: 1]

Orchidaceae establish symbiotic relationships with fungi in the Rhizoctonia group, resulting in interactions beneficial to both organisms or in cell destruction in one of them (pathogenicity). Previous studies have focused mostly on terrestrial species with a few, preliminary studies, on epiphytes. To further our understanding of the molecular mechanisms involved in these symbioses, we evaluated the interaction between Oncidium sphacelatum Lindl. and the mycorrhizal fungus Thanatephorus sp. strain RG26 (isolated from a different orchid species) in vitro using morphometric and proteomic analyses. Evidence from the morphometric and microscopic analysis showed that the fungus promoted linear growth and differentiation of orchid protocorms during 98 days interaction. On day 63, protocorm development was evident, so we analyzed the physiological response of both organisms at that moment. Proteome results suggest that orchid development stimulated by the fungus apparently involves cell cycle proteins, purine recycling, ribosome biogenesis, energy metabolism, and secretion that were up-regulated in the orchid; whereas in the fungus, a high expression of proteins implicated in stress response, protein-protein interaction, and saccharides and protein biosynthesis were found in the symbiotic interaction. This is the first work reporting proteins differentially expressed in the epiphytic orchid-fungus interaction and will contribute to the search for molecular markers that will facilitate the study of this symbiosis in both wild orchids and those in danger of extinction.
Soil P reduces mycorrhizal colonization while favors fungal pathogens: observational and experimental evidence in Bipinnula (Orchidaceae)
DOI:10.1093/femsec/fiaa178 URL [本文引用: 1]
The uncultured microbial majority
Progress towards DNA barcoding of fungi
High specificity generally characterizes mycorrhizal association in rare lady’s slipper orchids, genus Cypripedium
DOI:10.1111/j.1365-294X.2005.02424.x URL [本文引用: 1]
In situ symbiotic seed germination, isolation and identification of effective mycorrhizal fungus in Paphiopedilum hirsutissimum (Orchidaceae)
Media screening on seeds germination of Cremastra appendiculata
Ex-situ symbiotic seed germination of Dendrobium catenatum
DOI:10.5846/stxb URL
Correlation between vertical distribution of three Cypripedium species and composition of orchid mycorrhizal fungal community
Identification and symbiotic ability of Psathyrellaceae fungi isolated from a photosynthetic orchid, Cremastra appendiculata (Orchidaceae)
DOI:10.3732/ajb.1300099 URL [本文引用: 3]
Six new record species of Orchidaceae to Qinling Mountain
Fungal and bacterial communities in the rhizosphere of Pinus tabulaeformis related to the restoration of plantations and natural secondary forests in the Loess Plateau, Northwest China
Biological control of tobacco bacterial wilt using Trichoderma harzianum, amended bioorganic fertilizer and the arbuscular mycorrhizal fungi Glomus mosseae
DOI:10.1016/j.biocontrol.2015.10.013 URL [本文引用: 1]
Effect of endophyte extract on micropropagation of Cremastra appendiculata (D. Don.) Makino (Orchidaceae)
Progress of fungal DNA barcode
Establishment of a rice enhancer trap mutant library by T-DNA insertion