一氧化氮(NO)是生物细胞中的活性小分子,微溶于水、具有脂溶性,能很容易地穿过生物膜扩散,在细胞保护、耐药性和细胞生长发育等方面都具有重要调节功能(Krasuska et al. 2015;张容容 2017)。生物细胞中一般具有响应NO的应答系统,同时,NO还可能是一些重要酶如蛋白磷酸酶的上游信号分子,也会间接影响特定调节蛋白,因此,NO可以作为信号分子参与调节生物体的一些生理变化和生长发育过程,是细胞中的重要信号分子(徐茂军 2007;Li et al. 2012;Lagoda et al. 2014;Krasuska et al. 2015;代欢欢等 2020;王晓玲 2020)。
苯丙氨酸解氨酶(phenylalanine ammonia lyase,PAL)既是生物细胞内苯丙烷类代谢的关键酶,也是细胞响应外界化合物或对逆境胁迫响应的一个关键作用酶(Huang et al. 2010;张容容 2017;Arafa et al. 2020;王晓玲 2020)。细胞色素P450(CytochromeP450,CYP450)是灵芝细胞中与三萜化合物合成有关的重要酶系(Chen et al. 2012;陈慧等 2015)。
前期研究表明,长链脂肪酸9,10-环甲基十七烷酸可诱导灵芝菌体中三萜酸的合成(Zhang et al. 2020),本研究拟评估9,10-CMA在NO分子介导下,对灵芝中NO释放、CYP450和PAL激活的相关影响,初步分析9,10-CMA促进三萜酸合成的信号途径机制。并进一步探究9,10-CMA对灵芝细胞中三萜酸合成的关键酶基因表达的影响。
1 材料与方法
1.1 主要材料和试剂
1.1.1 灵芝菌种:灵芝菌株Ganoderma lingzhi SCIM 1006,由戴玉成教授采自湖南长沙市岳麓山。
1.1.2 主要试剂:9,10-环甲基十七烷酸(Cis- 9,10-methylenehexadecanoic acid,9,10-CMA,美国Matreya公司),PAL抑制剂L-2-氨基氧-3-苯基丙酸(L-AOPP,Sigma公司)、NO供体硝普钠(sodium nitroprusside,SNP,Sigma公司)、NO淬灭剂羟基-2-苯基-4,4,5,5-四咪唑-1-羟基-3-氧化物(cPITO,Sigma公司)及NOS(NO合酶)抑制剂Nʹ-硝基-L-精氨酸(L-NNA,Sigma公司)。三萜合成酶基因表达分析相关试剂盒(伯乐公司)。
1.2 灵芝发酵
1.2.1 种子培养:从斜面或者平皿中挑取灵芝菌种,接到80mL种子培养基中,在30℃、260r/min的摇床中恒温培养8d。种子发酵培养液(g/L):蛋白胨5.0、葡萄糖40.0、MgSO4 0.45、KH2PO4 0.75、VB1 0.01(张容容 2017)。
1.2.2 发酵:将种子液接种于250mL三角瓶中,然后于30℃、260r/min下发酵8d。接种量12%(V/V),每组试验3个平行。培养液(g/L):蛋白胨4.5、葡萄糖42.0、MgSO4·7H2O 0.45、KH2PO4 0.75、维生素B1 0.01。在发酵初始72h内pH值控制为5.07,72h后控制为4.37(张容容 2017)。
1.3 NO诱导及处理方法
以0.5mmol/L浓度将L-AOPP、SNP、cPITO、L,依据试验要求添加到灵芝发酵液中,对照组中用等体积的蒸馏水代替。在发酵第4天时,开始用9,10-CMA(0.15g/L)诱导。以上抑制剂在诱导处理前添加(张容容 2017)。平行样处理(3次)。
1.4 相关分析方法
1.4.1 NO浓度测定:采用Greiss法测定(Keser et al. 2013;王晓玲 2020)。
1.4.2 PAL活性分析:参照宛国伟等(2007)和王晓玲(2020)的方法进行。
1.4.3 CYP450含量分析:采用CO还原差分光谱法(Omura & Sato 1964;Dong et al. 2018)。
1.4.4三萜酸定量:以熊果酸为标准品,用比色法定量(Fang & Zhong 2002;Wang et al. 2016;王晓玲 2020)。
1.4.5 三萜合成重要酶基因表达分析:分别于灵芝培养的第2、4、6、7、8、9天收集菌丝。RNA的提取使用Omega植物RNA提取试剂盒提取,分析CYP450 Monooxygenases CYP512a2基因和CYP5150L8基因时,通过SYBR Premix Ex TaqTMⅡ(TaKaRa)试剂盒分析基因的表达量(Xu et al. 2019)。其他基因以TransScript All‐in‐One First‐Strand cDNA Synthesis SuperMix for qPCR试剂盒和伯乐试剂盒进行分析(内参基因GADPH)。转录水平的检测采用荧光定量PCR 2-ΔΔCT(Livak)法(Xu et al. 2019;王晓玲 2020)。
1.4.6 数理统计:用Origin 9.0软件对数据进行处理,每个样品设置3组平行。差异显著性分析:用t检验分析时,用星号个数表示与对照组相比的差异显著程度(*P<0.05;**P<0.01)。用Duncan’s multiple range test方法分析各处理组间的差异。
2 结果与分析
2.1 9,10-CMA对灵芝中NO浓度和PAL、CYP450活性的影响
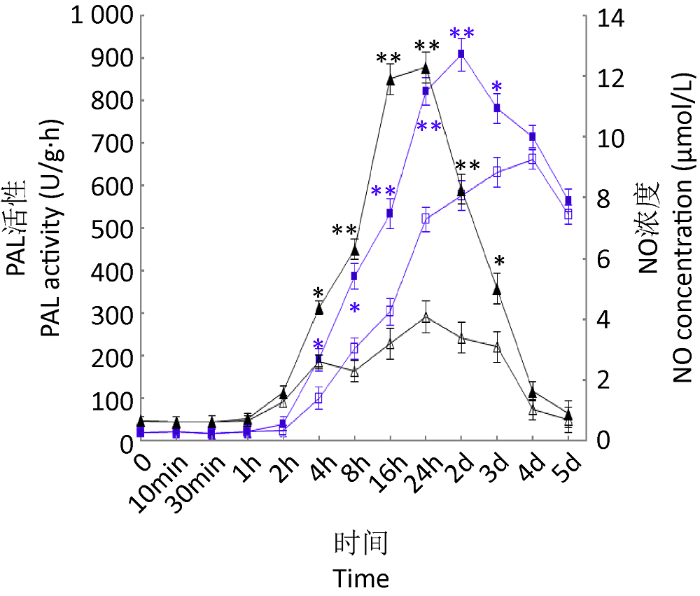
当加入9,10-CMA 2h后,灵芝细胞中的NO含量显著升高,并于16-24h时达到最大浓度;同时,在9,10-CMA处理2d左右后,PAL活性达到最高,而对照组在第4天达到峰值(图1),并且明显小于诱导组的峰值(P<0.01)。
图1
图1
9,10-CMA对灵芝细胞NO含量和PAL活性的影响
▲处理组NO浓度;△对照组NO浓度;■处理组PAL活性;□对照组PAL活性;*表示在每个时间点与未处理样本的显著差异程度(*P<0.05,**P<0.01). 下同
Fig. 1
The impact of 9,10-CMA on NO level and PAL activity.
▲ NO concentration in treatment group; △ NO concentration in control; ■ PAL activity in treatment group; □ PAL activity in control. Asterisks indicates significant differences from no 9,10-CMA treatment samples at each time point (*P<0.05, **P<0.01). The same below.
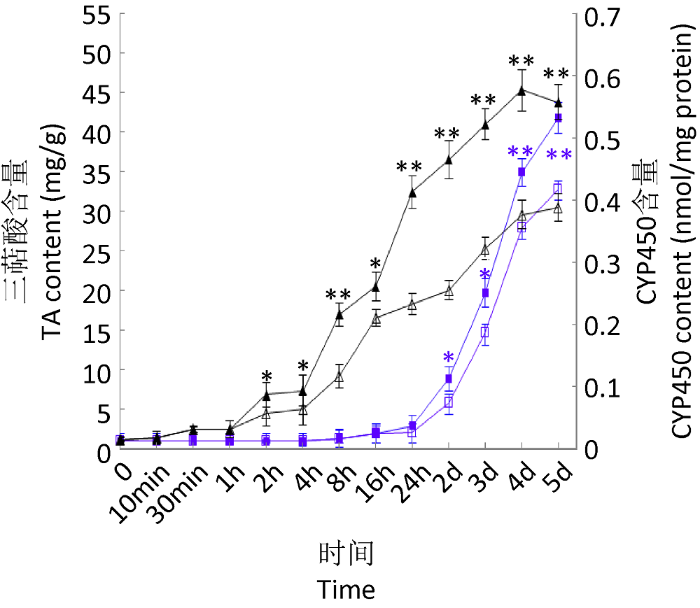
图2
图2
9,10-CMA对灵芝细胞产三萜酸和CYP450的影响
▲处理组CYP450含量;△对照组CYP450含量;■处理组三萜酸含量;□对照组三萜酸 含量
Fig. 2
The impact of 9,10-CMA on triterpenoid acid (TA) and CYP450 content.
▲ CYP450 content in treatment group; △ CYP450 content in control; ■ TA content in treatment group; □ TA content in control.
2.2 NO是9,10-CMA诱导CYP450和PAL活化的上游信号分子
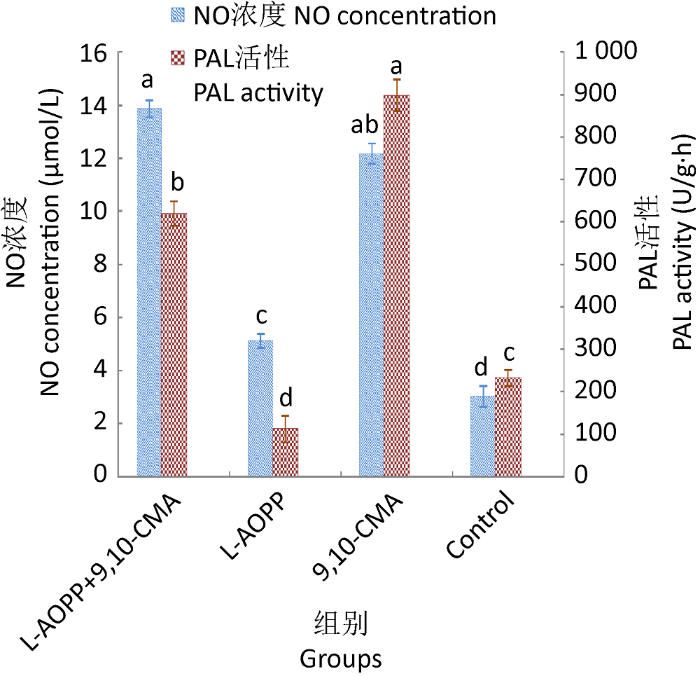
为进一步证明NO是9,10-CMA引起CYP450产生与PAL活化的上游信号分子,使用PAL抑制剂L-2-氨基氧-3-苯基丙酸(L-AOPP)处理菌丝体细胞(张容容 2017)。
图3
图3
L-AOPP对9,10-CMA诱导灵芝细胞PAL活性和NO浓度的影响
各指标各组间用不同字母标记表示有差异;相同字母者,表示差异不显著(邓肯多重范围检验,P<0.05). 下同
Fig. 3
The impact of L-AOPP on NO level and PAL activity of Ganoderma lingzhi induced by 9,10-CMA in liquid medium.
There were differences among the groups marked with different letters; the same letter means that the difference is not significant (Duncan’s multiple range test, P<0.05). The same below.
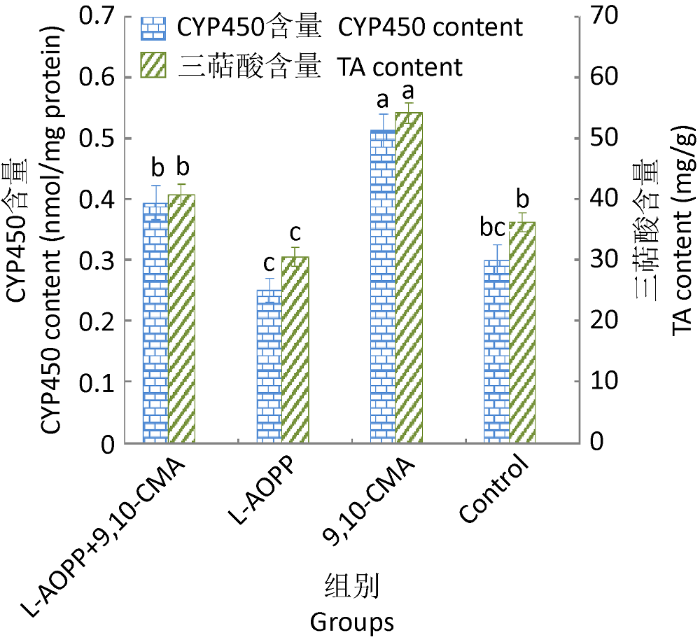
L-AOPP+9,10-CMA组中三萜酸和CYP450含量低于9,10-CMA组,但高于L-AOPP组和对照组(P<0.05);L-AOPP组与对照组相比,三萜酸和CYP450的含量降低较明显(P<0.05,图4),进一步表明L-AOPP抑制9,10-CMA诱导灵芝三萜酸合成和CYP450活性,但并不完全抑制。
图4
图4
L-AOPP对9,10-CMA诱导灵芝细胞产CYP450和三萜酸的影响
Fig. 4
The impact of L-AOPP on CYP450 content of Ganoderma lingzhi cells with 9,10-CMA in liquid culture.
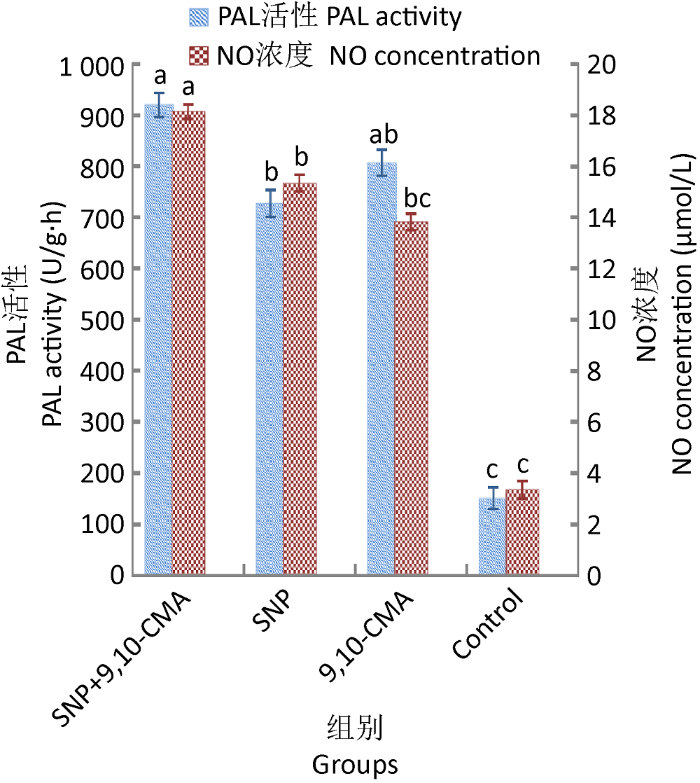
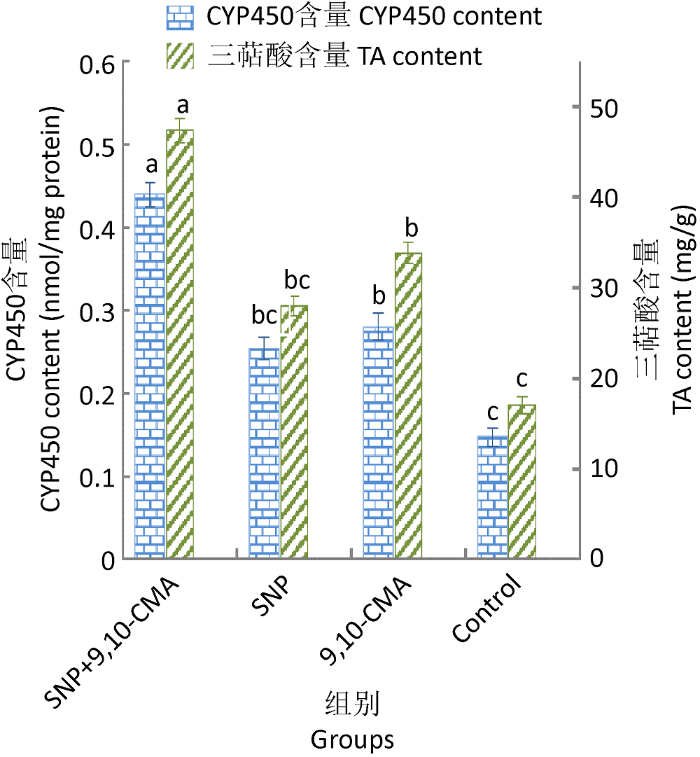
2.3 NO供体对CYP450含量和PAL活性的影响
2.3.1 NO不是灵芝三萜合成途径中上游的唯一信号分子:SNP是常用的NO供体,试验了SNP对灵芝中CYP450含量及PAL活性的影响(张容容 2017)。
图5
图5
SNP对9,10-CMA诱导灵芝细胞PAL活性和NO浓度的影响
Fig. 5
The impact of sodium nitroprusside (SNP) on NO concentration and PAL activity of Ganoderma lingzhi induced by 9,10-CMA in liquid medium.
图6
图6
SNP对9,10-CMA诱导灵芝细胞产三萜酸和CYP450的影响
Fig. 6
The impact of sodium nitroprusside (SNP) on TA and CYP450 levels of Ganoderma lingzhi induced by 9,10-CMA in liquid medium.
2.3.2 9,10-CMA与NO协同作用的分析:9,10- CMA+SNP共处理组的NO浓度、CYP450含量和PAL活性均显著高于SNP处理组、9,10-CMA诱导组和对照组,表明9,10-CMA与NO能共同参与CYP450产生和PAL活化,协同作用并诱导灵芝三萜的产生。
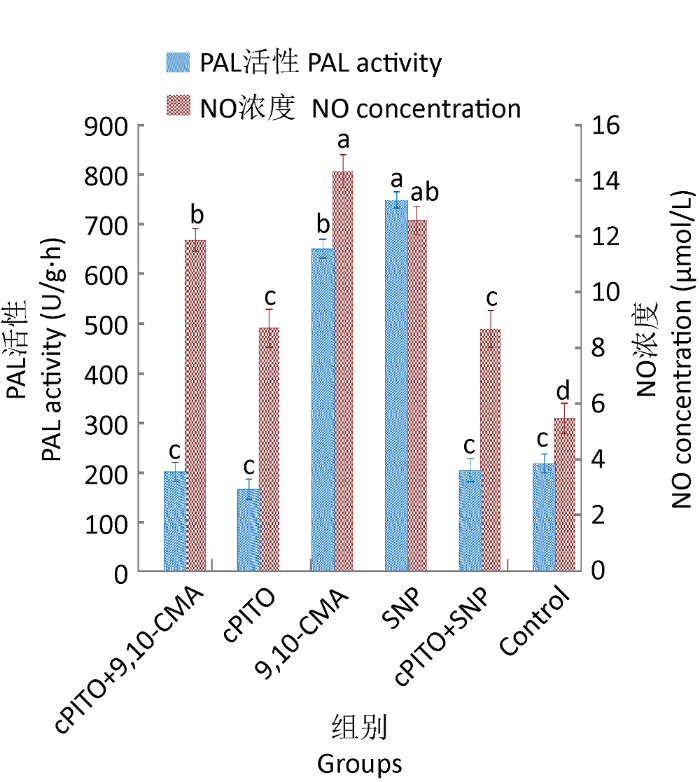
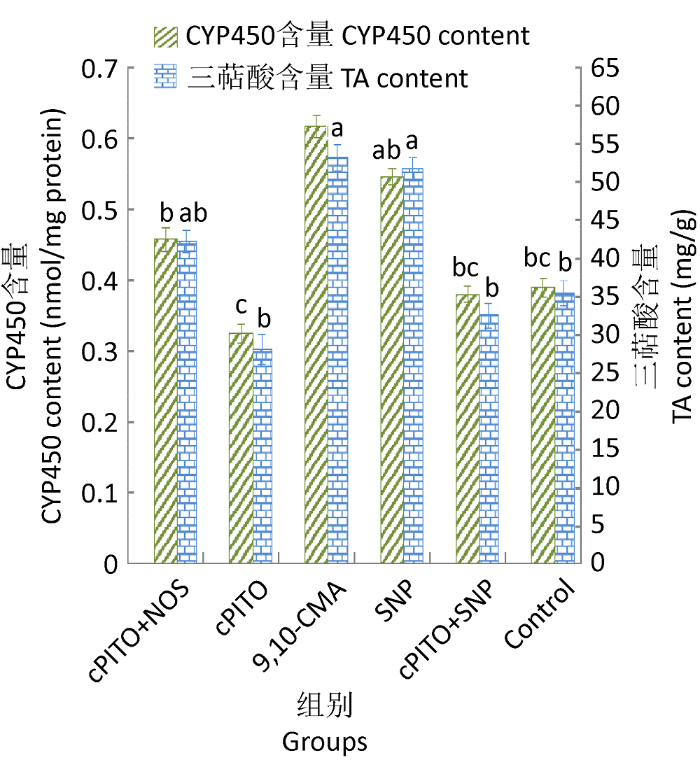
2.4 L-NNA和cPITO对灵芝三萜合成诱导的影响
试验了NO淬灭剂(cPITO)和NO合酶(NOS)抑制剂(L-NNA)对9,10-CMA诱导细胞中NO浓度、CYP450含量、PAL活性、三萜酸含量的影响,以验证NO是否是9,10-CMA诱导灵芝三萜合成的必须信号分子(张容容 2017)。
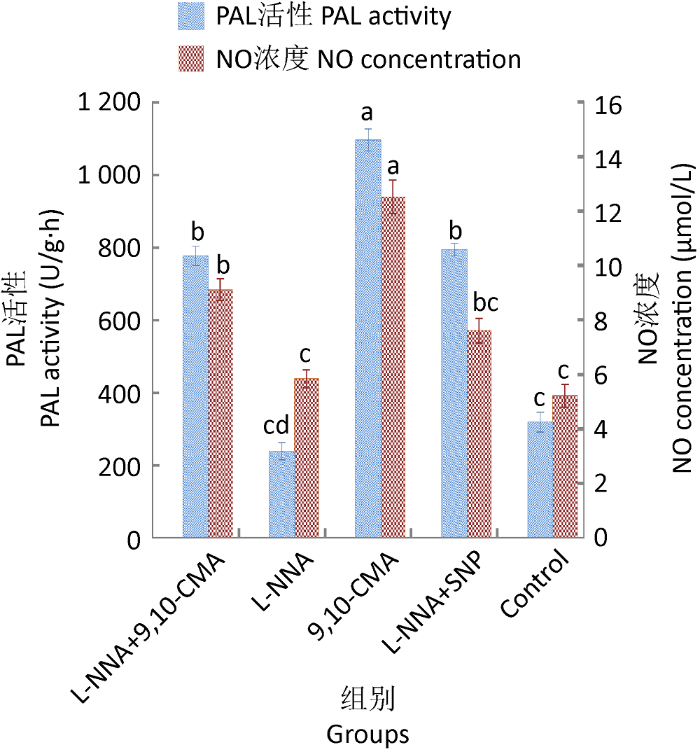
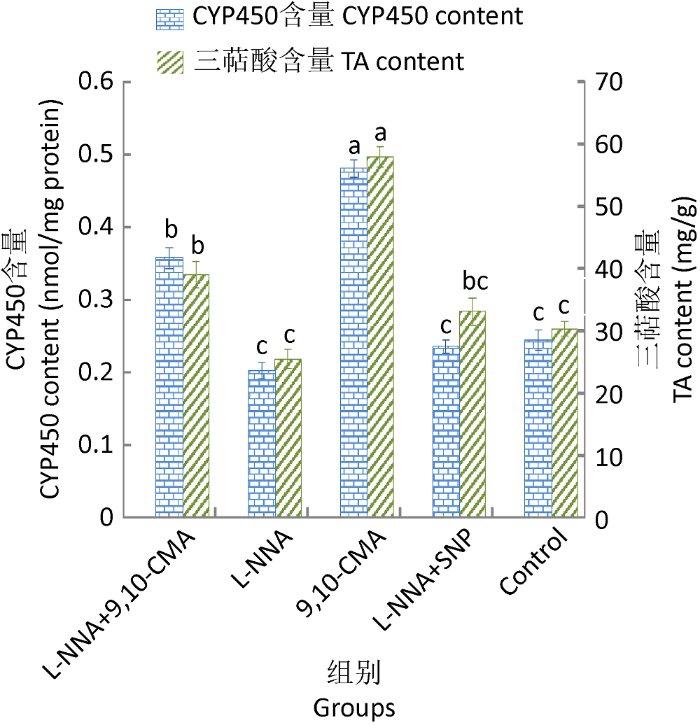
2.4.1 L-NNA对CYP450含量和PAL活化的影响:与L-NNA+9,10-CMA组相比,诱导组中NO浓度、CYP450含量和PAL活性均显著升高(图7),表明L-NNA能显著抑制灵芝中NO的释放,进而对CYP450和PAL活性产生影响;也提示9,10-CMA在诱导灵芝合成三萜之前CYP450和PAL活性对NO有依赖作用。在诱导过程中,NO 合酶(NOS)仍是产生NO的主要途径。
图7
图7
L-NNA对9,10-CMA诱导灵芝细胞中PAL活性和NO浓度的影响
Fig. 7
The impact of N-nitro-L-arginine (L-NNA) on NO level and PAL activity of Ganoderma lingzhi induced by 9,10-CMA in liquid culture.
L-NNA+9,10-CMA组与对照组相比,前者的NO含量大幅度升高(图8),这提示L-NNA 只能部分抑制9,10-CMA诱导释放的NO。另外,与对照组相比,L-NNA+9,10-CMA组中CYP450含量和PAL活性也有显著增加(P<0.05),进一步证实了9,10-CMA引发灵芝中CYP450产生与PAL活化需要NO这一信号分子。
图8
图8
L-NNA对9,10-CMA诱导灵芝细胞产CYP450和三萜酸的影响
Fig. 8
The impact of N-nitro-L-arginine (L-NNA) on TA and CYP450 levels of Ganoderma lingzhi induced by 9,10-CMA in liquid medium.
2.4.2 NO并不是9,10-CMA诱导灵芝三萜酸合成的必须信号:cPITO+SNP组、cPITO处理组与对照组中CYP450含量和NO浓度无明显差异(图9),即cPITO能有效清除SNP产生的NO,表示NO能引发SNP组中的CYP450含量增加和PAL活性升高。
图9
图9
cPITO对9,10-CMA诱导灵芝细胞中PAL活性和NO浓度的影响
Fig. 9
The impact of (cPITO) on NO level and PAL activity of Ganoderma lingzhi induced by 9,10-CMA in liquid medium.
cPITO+9,10-CMA组与9,10-CMA组相比,前者的NO浓度、CYP450含量、PAL活性和三萜酸含量都有大幅度降低(图9),表明可以通过外源添加cPITO明显清除9,10-CMA诱导产生的NO,进一步使CYP450含量和PAL活性降低,也说明9,10-CMA在诱导菌丝体细胞产生灵芝三萜之前CYP450含量和PAL活性仍依赖NO的量。
图10
图10
cPITO对9,10-CMA诱导灵芝细胞产三萜酸和CYP450的影响
Fig. 10
The impact of cPITO on TA and CYP450 levels of Ganoderma lingzhi induced by 9,10-CMA in liquid medium.
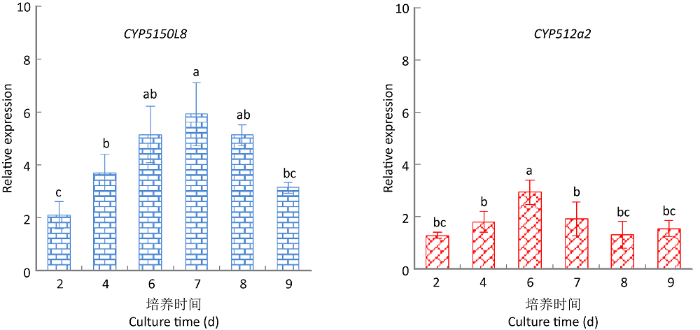
2.5 9,10-CMA诱导下三萜酸合成基因的表达变化
图11
图11
灵芝三萜酸重要合成酶CYP512a2和CYP5150L8基因在9,10-CMA干预下的动态表达变化
Fig. 11
Dynamic expression changes of key synthases genes CYP512a2 and CYP5150L8 in Ganoderma lingzhi under the intervention of 9,10-CMA.
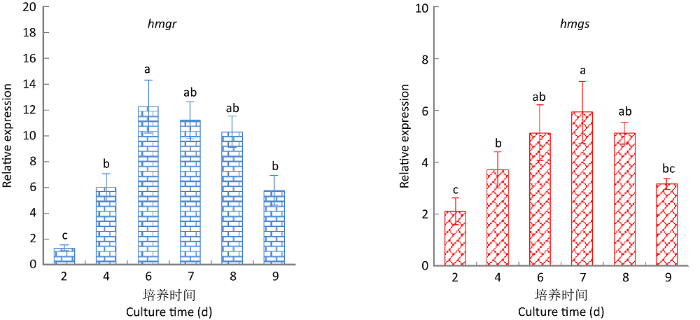
图12
图12
灵芝三萜酸重要合成酶HMGS和HMGR基因在9,10-CMA干预下的动态表达变化
Fig. 12
Dynamic expression changes of key synthases genes HMGS and HMGR in Ganoderma lingzhi under the intervention of 9,10-CMA.
图13
图13
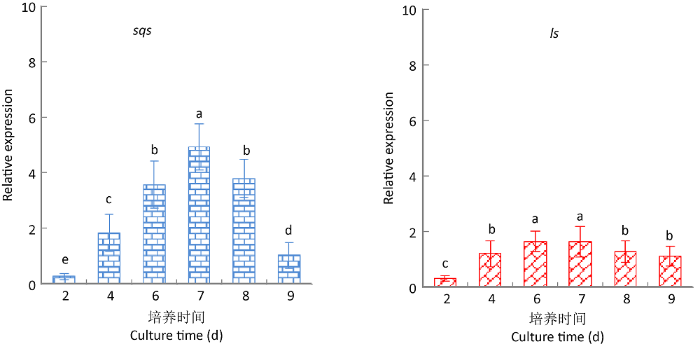
灵芝三萜酸重要合成酶LS和SQS基因在9,10-CMA干预下的动态表达变化
Fig. 13
Dynamic expression changes of key synthases genes LS and SQS in Ganoderma lingzhi under the intervention of 9,10-CMA.
实验结果表明,三萜酸合成的6种重要酶基因的转录水平都有不同幅度的上调,其中基因sqs、CYP5150L8、hmgr的上调最为显著,sqs和hmgr在第6天达到最大上调值,CYP5150L8在第7天达到最大上调值,上调量分别为对照组的4.93、12.27和5.92倍。
3 讨论
NO是介导细胞次生代谢产物合成的一种必需信号分子(徐茂军等 2004;Grun et al. 2006;Lagoda et al. 2014;Krasuska et al. 2015;代欢欢等 2020)。本研究中,NO作为苯丙氨酸代谢途径中的上游信号分子,它介导了9,10-CMA诱导灵芝菌丝体细胞中CYP450的产生和PAL的活化,但NO这种介导作用并不是9,10-CMA诱导作用的必需因素。本研究表明,在灵芝菌丝体细胞中除了NO信号分子外,可能还存在其他外界信号因子或者信号途径共同介导9,10-CMA促进CYP450和PAL的活性提高。因此,NO是9,10-CMA诱导灵芝细胞中CYP450产生和PAL活化的信号转导途径中所必要存在、但不是唯一存在的信号分子(王晓玲 2020)。
作者新近研究表明,薏苡仁油(CSO)可通过H2O2和NO两个信号分子介导,进而促进灵芝三萜酸的合成(Liu et al. 2019)。本研究表明,NO也可通过介导9,10-CMA的诱导作用而促进灵芝三萜酸的产生。Liu et al. (2018a,2018b)报道,在热胁迫下,合成灵芝三萜酸(灵芝酸)的过程中也需要NO和H2O2信号参与,有趣的是,NO虽然降低了热应激诱导下的三萜酸积累,但促进了CSO或9,10-CMA诱导下三萜酸积累(Liu et al. 2018a)。因此,在不同环境或诱导条件下,NO在目标代谢物生物合成中的调控机制可能不同。目前有关NO等信号分子在三萜酸生物合成中的介导/调控机制的研究还相对较少。
当外源物质或环境因子对细胞产生影响时,PAL作为应激酶,其活性便得到激活,引发细胞防御反应,并引起具防御作用的一些次生物质如三萜、黄酮类物质的产生(Huang et al. 2010;常志凯等 2016;Nag & Kumaria 2018;Arafa et al. 2020)。常志凯等(2016)报道,对白桦悬浮细胞体系进行高温胁迫后,再加入茉莉酸甲酯(MeJA),可能通过影响白桦细胞生长量、细胞活力、丙二醛量和防御酶(PAL)活性变化,调节三萜合成关键酶基因的表达,最终促进三萜物质的高效合成与积累。本研究中,灵芝细胞经9,10-CMA处理后可诱发NO释放、PAL和CYP450活化的相关生理响应,提示灵芝细胞在9,10-CMA处理后也激活了PAL且引起三萜合成量增加,但与常志凯等(2016)的研究一样,并没有PAL与三萜合成关联的直接证据。目前尚缺少PAL与三萜合成二者具体关系的文献。
目前已有研究显示,合成萜类化合物需从乙酰辅酶A(Acetyl CoA)以及被称为甲羟戊酸(MVA)的途径(MVP)开始,即三萜合成的前体是甲羟戊酸(陈慧等 2015;Dong et al. 2019)。现有研究表明,灵芝三萜与其他萜类化合物一样,也是由MVP开始合成的(Chen et al. 2012;陈慧等 2015),即从乙酰CoA开始经过一系列反应生成异戊烯焦磷酸酯(IPP),由IPP接着生成法尼基二磷酸(FPP),FPP是合成重要中间体鲨烯的底物,由角鲨烯合成酶(SQS)催化合成。然后经鲨烯环氧酶(SE)催化使鲨烯转变为灵芝三萜前体2,3-氧化鲨烯。2,3-氧化鲨烯环化酶(OSCs)作用于2,3-氧化鲨烯得到灵芝五环三萜类骨架;进一步通过羊毛甾醇合酶(LS)环化合成羊毛甾醇,并最终形成四环三萜类骨架(Chen et al. 2012;陈慧等 2015)。在上述三萜的复杂合成过程中,3‐羟基‐3‐甲基戊二酰辅酶A合酶(HMGS)、3‐羟基‐3‐甲基戊二酰辅酶A还原酶(HMGR)、甲羟戊酸激酶(MVK)、焦磷酸甲羟戊酸脱羧酶(MVD)、鲨烯环氧酶(SE)、鲨烯合成酶(SQS,合成鲨烯的酶)、法尼基二磷酸合成酶(FPS,合成FPP)、2,3-氧化鲨烯环化酶(OSCs)、羊毛甾醇合成酶(LS),以及细胞色素P450等合成酶是影响灵芝三萜合成的关键酶(Chen et al. 2012;陈慧等 2015;Dong et al. 2019;Xu et al. 2019)。本研究通过荧光定量PCR检测了其中6个重要酶的基因动态表达情况,结果表明,与灵芝三萜酸合成相关的鲨烯合酶基因sqs、细胞色素P450单加氧酶CYP5150L8基因、3‐羟基‐3‐甲基戊二酰辅酶A还原酶基因hmgr的上调幅度最高,表明3个合成酶可能是在9,10-CMA诱导灵芝合成三萜的过程中起重要作用的合成酶,具体响应基因/蛋白的鉴定和相关功能有待进一步研究。
参考文献
Purification and characterization of anabaena flos-aquae phenylalanine ammonia-lyase as a novel approach for myristicin biotransformation
DOI:10.4014/jmb.1908.08009 URL [本文引用: 2]
Effect of MeJA combined with high temperature stress in treatment for accumulation of triterpenoids in birch suspension cells
Biosynthesis and fermentation control of triterpenoids from Ganoderma lingzhi
Genome sequence of the model medicinal mushroom Ganoderma lucidum
Effects of exogenous NO on nitrogen metabolism and secondary metabolism of belladonna under salt stress
Site-directed mutagenesis of cytochrome P450 2D6 and 2C19 enzymes: expression and spectral characterization of naturally occurring allelic variants
DOI:10.1007/s12010-018-2728-0 URL [本文引用: 1]
High oxygen treatments enhance the contents of phenolic compound and ganoderic acid, and the antioxidant and DNA damage protective activities of Ganoderma lingzhi fruiting body
Submerged fermentation of higher fungus Ganoderma lucidum for production of valuable bioactive metabolites— ganoderic acid and polysaccharide
Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress
DOI:10.1104/pp.110.157370 URL [本文引用: 2]
Brain nitric oxide metabolites in rats preselected for nicotine preference and intake
DOI:10.1016/j.neulet.2013.04.027 URL [本文引用: 1]
Switch from heterotrophy to autotrophy of apple cotyledons depends on NO signal
DOI:10.1007/s00425-015-2361-x
PMID:26186967
[本文引用: 3]

NO accelerates transition of germinated embryos from heterotrophy to autotrophy by stimulation of chloroplasts maturation. NO-mediated autotrophy of apple seedlings correlates to increased content of RuBisCO small subunit and improvement of parameters of chlorophyll a fluorescence. Nitric oxide (NO) acts as signaling molecule involved in regulation of various physiological processes in plants, although its involvement in cotyledons greening is poorly recognized. To identify the importance of NO signal for plant growth and development we investigated the effects of short-term application of NO at various developmental stages of seedlings of apple (Malus domestica Borkh.) on cotyledons' chlorophyll a to b ratio, chlorophyll a fluorescence, photosynthetic activity, carbohydrates and RuBisCO both subunits content. NO-dependent biochemical alterations were linked to cytological observation of developing plastids in cotyledons of apple plants. Abnormal plantlets developing from dormant apple embryos are characterized by anatomical malformations of cotyledons. Short-term pre-treatment with NO of isolated embryos or seedlings with developmental anomalies resulted in formation of plants with cotyledons of equal size and chlorophyll content; these responses were blocked by NO scavenger. NO independently of time point of application accelerated embryos transition from heterotrophy to autotrophy by stimulation of photosynthetic activity, improvement of parameters of chlorophyll a fluorescence (F v/F m, F v/F 0) and increased content of RuBisCO small subunit. Further analysis showed that NO application modified glucose and hydrogen peroxide concentration in cotyledons. Beneficial effect of NO on development of seedlings without any abnormalities was manifested at ultrastructural level by decline in amount of proplastids and induction of formation and maturation of chloroplasts. Our data suggest that progress of autotrophy of young seedlings is governed by NO acting as stimulator of chloroplast-to-nucleus signaling.
Sustained nitric oxide (NO)-releasing compound reverses dysregulated NO signal transduction in priapism
DOI:10.1096/fj.13-228817
PMID:24076963
[本文引用: 2]

We evaluated the therapeutic potential of a sustained nitric oxide (NO)-releasing compound to correct the molecular hallmarks and pathophysiology of priapism, an important but poorly characterized erectile disorder. 1,5-Bis-(dihexyl-N-nitrosoamino)-2,4-dinitrobenzene (C6') and an inactive form of the compound [1,5-bis-(dihexylamino)-2,4-dinitrobenzene (C6)] were tested in neuronal cell cultures and penile lysates for NO release (Griess assay) and biological activity (cGMP production). The effect of local depot C6' or C6 was evaluated in mice with a priapic phenotype due to double neuronal and endothelial NO synthase deletion (dNOS(-/-)) or human sickle hemoglobin transgenic expression (Sickle). Changes in NO signaling molecules and reactive oxygen species (ROS) surrogates were assessed by Western blot. The physiological response after C6' treatment was assessed using an established model of electrically stimulated penile erection. C6' generated NO, increased cGMP, and dose dependently increased NO metabolites. C6' treatment reversed abnormalities in key penile erection signaling molecules, including phosphodiesterase type 5, phosphorylated endothelial nitric oxide synthase, and phosphorylated vasodilator-stimulated phosphoprotein. In Sickle mice, C6' also attenuated the increased ROS markers gp91(phox), 4-hydroxynonenal, and 3-nitrotyrosine. Finally, C6' corrected the excessive priapic erection response of dNOS(-/-) mice. Exogenous sustained NO release from C6' corrects pathological erectile signaling in mouse models of priapism and suggests novel approaches to human therapy.
Modulated expression of genes associated with NO signal transduction contributes to the cholesterol-lowering effect of electro-acupuncture
DOI:10.1007/s10529-012-0892-9 URL [本文引用: 1]
Cross talk between nitric oxide and calcium-calmodulin regulates ganoderic acid biosynthesis in Ganoderma lucidum under heat stress
Heat stress-induced reactive oxygen species participate in the regulation of HSP expression, hyphal branching and ganoderic acid biosynthesis in Ganoderma lucidum
DOI:10.1016/j.micres.2018.02.006 URL [本文引用: 1]
Interdependent nitric oxide and hydrogen peroxide independently regulate the coix seed oil induced triterpene acids accumulation in Ganoderma lingzhi
DOI:10.1080/00275514.2019.1615816 URL [本文引用: 1]
In silico characterization and transcriptional modulation of phenylalanine ammonia lyase (PAL) by abiotic stresses in the medicinal orchid Vanda coerulea Griff. ex Lindl
DOI:10.1016/j.phytochem.2018.09.012 URL [本文引用: 1]
The carbon monoxide- binding pigment of liver microsomes. ii. solubilization, purification, and properties
DOI:10.1016/S0021-9258(20)82245-5 URL [本文引用: 1]
Effects of culture conditions on phenylalanine ammonia lyase and polyphenol oxidase activities of Salvia miltiorrhiza root in vitro
Enhanced triterpene acid production by Ganoderma lucidum using a feeding stimulus integrated with a two-stage pH-control strategy
Fermentation strategies and related mechanism analysis of triterpenoid acid production by induction in submerged fermentation of new strains of Ganoderma lingzhi
Enhanced production of individual ganoderic acids by integrating Vitreoscilla haemoglobin expression and calcium ion induction in liquid static cultures of Ganoderma lingzhi
DOI:10.1111/mbt2.v12.6 URL [本文引用: 3]
Nitric oxide participates in the promotion of fungal elicitors on PAL activation and taxol biosynthesis in Taxus suspension cells
Nitric oxide: a possible key node in signal transduction network of plant cell secondary metabolism
Kinetic characteristics of triterpene acid synthesised by submerged fermentation of Ganoderma lingzhi under 9,10-methylene hexadecanoic acid intervention
The signal transduction mechanism of NO and H2O2 involved in the synthesis of Ganoderma lingzhi triterpenoids by coix seed oil
9,10-环甲基十七烷酸干预下灵芝深层发酵合成三萜酸的动力学特征