 PDF(605 KB)
PDF(605 KB)


 PDF(605 KB)
PDF(605 KB)
 PDF(605 KB)
PDF(605 KB)
地衣及其内生真菌活性次级代谢产物研究进展
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Research progress on bioactive secondary metabolites of lichens and endolichenic fungi
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}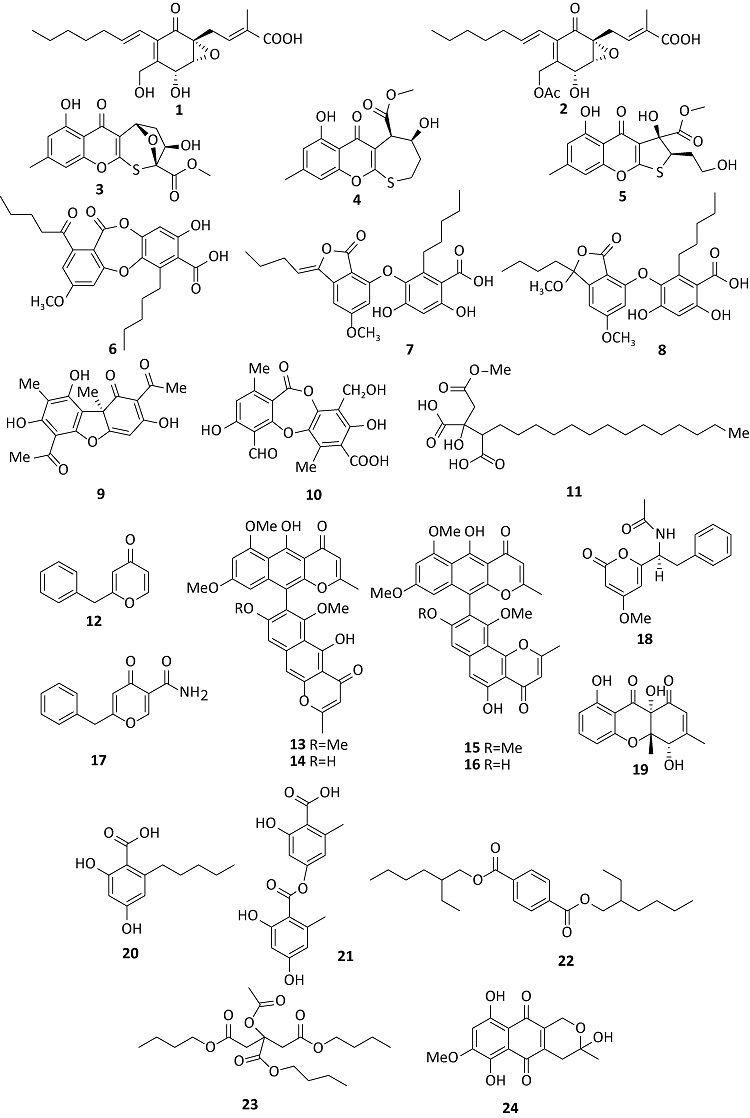
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |