 PDF(818 KB)
PDF(818 KB)


西北沙区梭梭根系深色有隔内生真菌等微生物时空分布及对根际土壤环境的响应
赵昕,高慧利,龙俊萌,刘燕霞,李夏,贺学礼
菌物学报 ›› 2021, Vol. 40 ›› Issue (10) : 2716-2734.
 PDF(818 KB)
PDF(818 KB)
 PDF(818 KB)
PDF(818 KB)
西北沙区梭梭根系深色有隔内生真菌等微生物时空分布及对根际土壤环境的响应
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Spatial and temporal distribution and the response to rhizosphere soil environment of dark septate endophyte and other microorganisms in roots of Haloxylon ammodendron in sand area of Northwest China
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}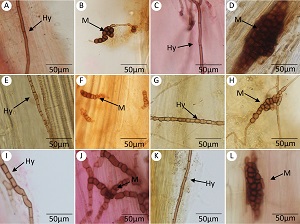
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |