 PDF(482 KB)
PDF(482 KB)


 PDF(482 KB)
PDF(482 KB)
 PDF(482 KB)
PDF(482 KB)
藏羚羊和藏野驴粪便真菌多样性比较研究
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Comparative study on fecal fungal diversity between Tibetan antelope and Tibetan wild ass
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}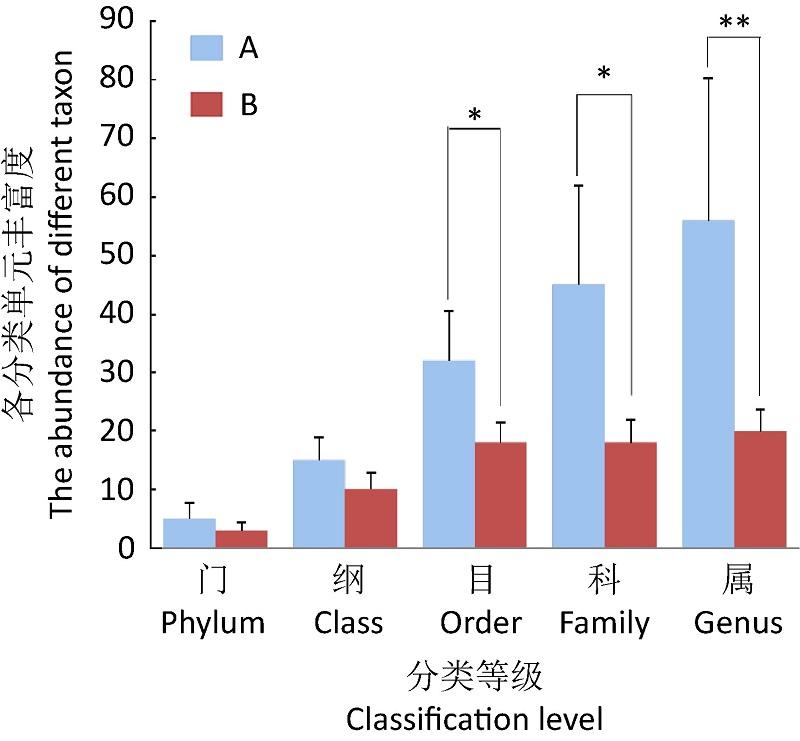
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |