 PDF(876 KB)
PDF(876 KB)


 PDF(876 KB)
PDF(876 KB)
 PDF(876 KB)
PDF(876 KB)
响应面法优化六妹羊肚菌边角料中麦角硫因的提取工艺
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Optimization of extraction process of ergothioneine from Morchella sextelata scraps by response surface methodology
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}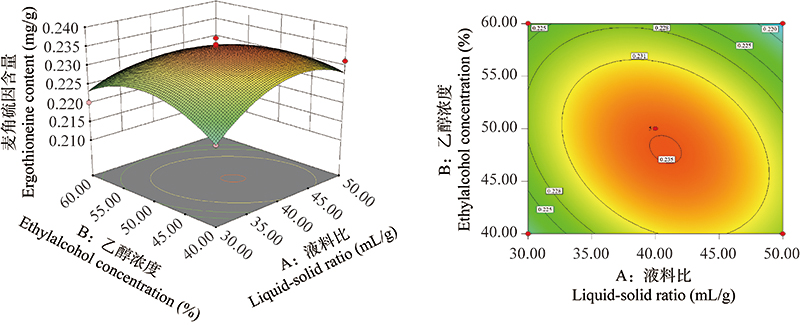
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |