 PDF(940 KB)
PDF(940 KB)


 PDF(940 KB)
PDF(940 KB)
 PDF(940 KB)
PDF(940 KB)
糙皮侧耳AQPs基因家族鉴定与表达分析
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Identification and expression analyses of the aquaporin gene family in Pleurotus ostreatus
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}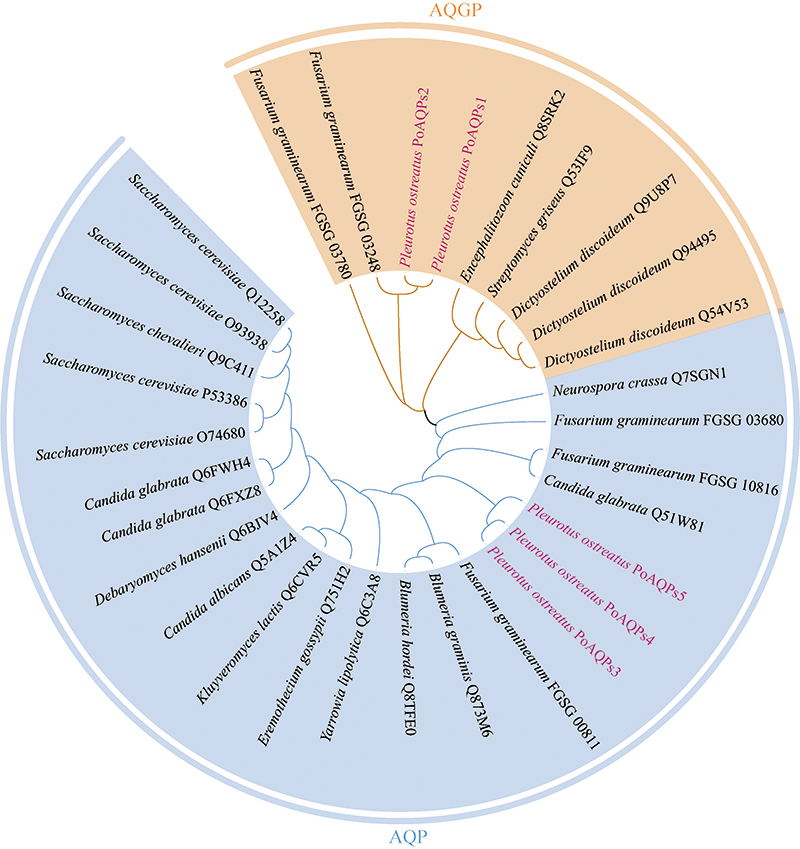
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |