 PDF(604 KB)
PDF(604 KB)


 PDF(604 KB)
PDF(604 KB)
 PDF(604 KB)
PDF(604 KB)
碳源对玫烟色虫草菌丝生长、芽生孢子产生及其耐热性的影响
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Effects of carbon sources on mycelial growth, blastospore production and thermotolerance of Cordyceps fumosorosea
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}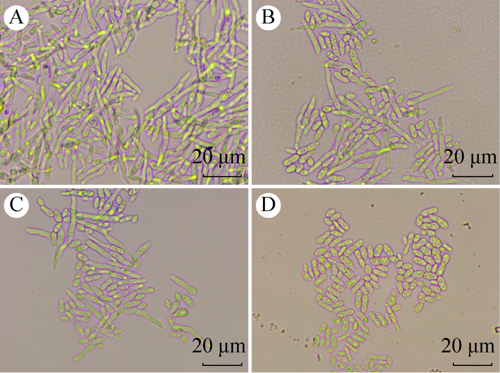
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |