 PDF(1801 KB)
PDF(1801 KB)


 PDF(1801 KB)
PDF(1801 KB)
 PDF(1801 KB)
PDF(1801 KB)
四种桑黄类真菌挥发性风味成分分析
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Analyses of volatile flavor components in four sanghuang fungi
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}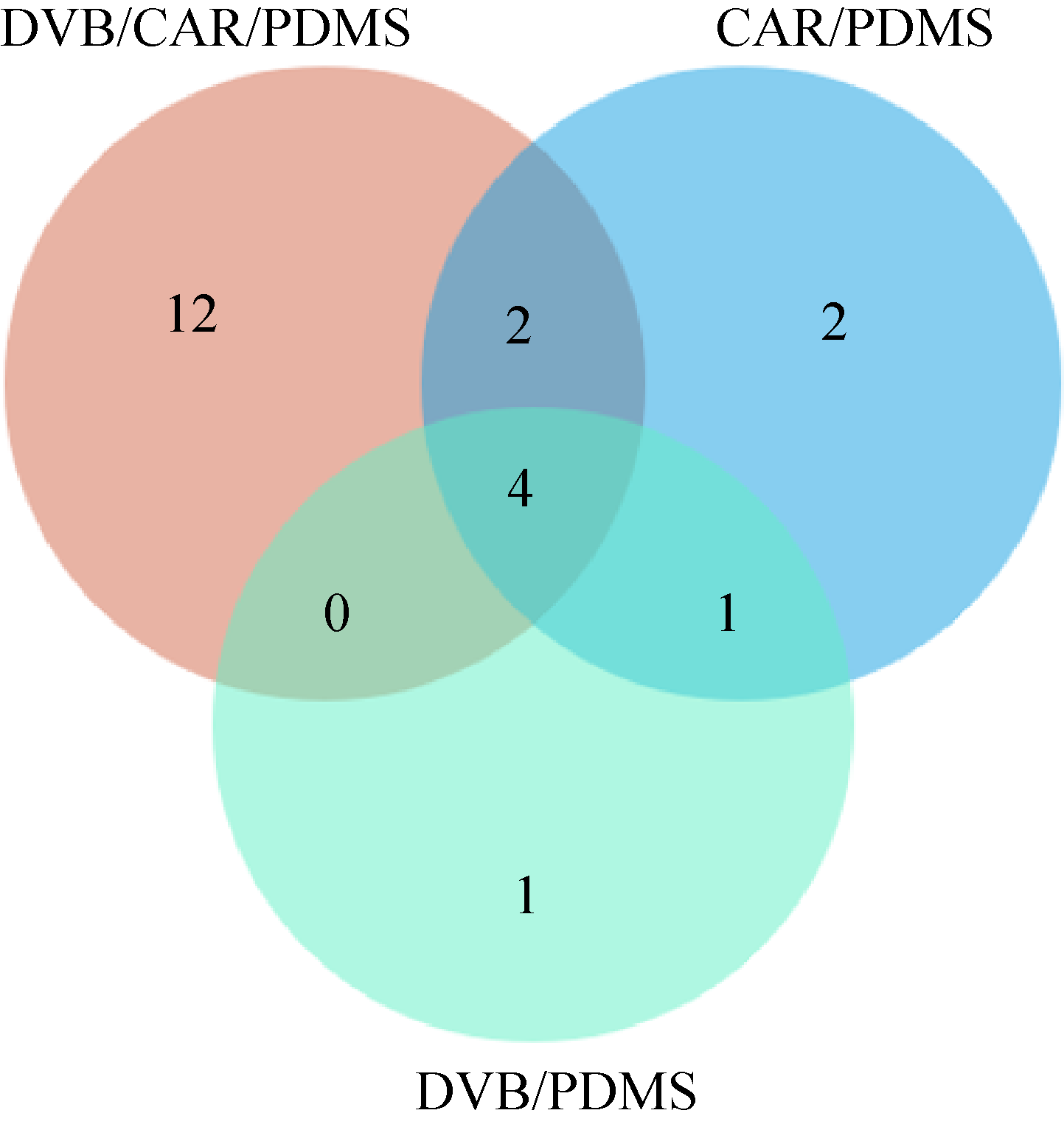
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |