由于受到全球气候变化和人类活动等影响,土地荒漠化现象日益严重(Tang et al. 2016)。荒漠化防治已成为实现经济和生态环境可持续发展的关键所在。在长期进化历程中,荒漠生态系统形成了具有典型生态特征的地带性植被,这些植被类型是荒漠生态系统的主要生产力来源。同时,植物在生态适应过程中,还通过与内生真菌建立功能共生体的调节机制来响应气候变化和环境胁迫(Wehner et al. 2013;Ali et al. 2018)。其中,深色有隔内生真菌(dark septate endophyte,DSE)作为荒漠化过程中非常重要的一类根系真菌,是联接地上和地下生态系统物质传输的桥梁。
DSE真菌通常被定义为植物根组织细胞内或细胞间隙的一群子囊菌和无性型真菌,可在不引起植物病症的情况下生长于植物根系表皮、皮层及维管组织细胞,形成颜色较深、有明显隔膜的菌丝及微菌核结构(Jummpponen & Trappe 1998;Addy et al. 2005;Mandyam et al. 2010)。这类真菌已被报道具有广泛的宿主和生态分布范围,其宿主植物超过114科320属近600种(Jumpponen & Trappe 1998),分布范围从温带、热带(Massenssini et al. 2014;Bonfim et al. 2016),到荒漠(张雪等 2019)、极地、高山(Kauppinen et al. 2014)等多种生态系统,尤其在干旱等极端环境的植物根系上有较高定殖率。研究表明,这类真菌群体能够通过赋予植物多种(非)生物胁迫耐受性,增强宿主分解氮、磷等化合物的能力,促进植物根系从土壤中吸收水分和养分(毕银丽和解琳琳 2021)等以提高宿主生态适应性。因此,荒漠植物与真菌相互关系的构建是荒漠生物学进程的重要部分,揭示植物-真菌-环境在不同时空尺度的相互作用能够深刻理解荒漠生态系统发生过程和演变规律,为荒漠生态系统稳定性发展和生态保护提供对策与依据。
近年来,国内外学者已在干旱生境的DSE真菌物种多样性及生态功能方面开展了相关研究。结果表明,DSE真菌存在于多种荒漠或旱区植物根系,并对宿主生长及抗旱性有益。如Barrow(2003)对美国半干旱草原格兰马草属植物调查发现,这些植物根系均有DSE真菌定殖,推测从植物根部延伸出的DSE真菌菌丝能够帮助共生植物在干旱环境中维持水分和养分运输,提高其抗旱性。Porras-Alfaro et al.(2008)在水分胁迫下接种5种DSE真菌至荒漠禾草发现,其中4种DSE真菌表现出对禾草宿主生长有益。然而,目前关于DSE真菌自然生境分布的研究多集中于植物是否带菌等调查,缺乏对其分布格局的形成机制和驱动因子的系统研究。据报道,土壤生物多样性和理化性质能极大程度影响植物内生真菌的定殖(Escudero & Mendoza 2005;Adil et al. 2017)。由于荒漠生态系统复杂多变的恶劣环境,DSE真菌的定殖和分布势必会受自然环境的整体影响(Adil et al. 2017)。Rayment et al.(2020)研究发现植物内生真菌的定殖会随生物或非生物等环境因素而变化以减少宿主植物的压力。因此,我们推测DSE真菌对植物根际土壤环境具有改善作用,也可能对植物生长及群落构建产生深远影响(Barrow 2003;张海涵 2011;Santos et al. 2017)。另外,真菌-植物共生关系在不同生长阶段的差异可能随季节变化而间接影响真菌定殖水平(Rayment et al. 2020)。如Mandyam & Jumpponen(2008)通过调查不同季节草原生态系统发现C3、C4植物内生真菌定殖率变化与植物物候和植物种类密切相关。因此在研究DSE真菌定殖影响因素时不同生长阶段的DSE-植物共生关系也应作考虑。
梭梭Haloxylon ammodendron,属黎科超旱生灌木状小乔木(徐高兴等 2019),是荒漠地区植被恢复的先锋树种(Enkhchimeg et al. 2020),在长期进化过程中形成了与干旱生境相适应的形态及生理特征,被广泛应用于荒漠绿化工程(Yang et al. 2020)。梭梭叶退化,主侧根发达,强大的地下根系系统不仅维持其在荒漠生境的生存,同时也为根际共生菌提供良好的栖息地(姚丹等 2020)。目前关于梭梭共生微生物的研究多集中于细菌方面,而对真菌的报道较少。在长期稳定共存的进化历程中,DSE真菌作为荒漠生境重要的一类共生菌,对梭梭的生长发育可能发挥着重要作用。因此,在将DSE真菌应用于荒漠植被恢复之前,系统研究荒漠生境中梭梭根系DSE真菌的分布和定殖规律至关重要。
地下根系系统将植物和土壤紧密连接,使根系影响土壤环境的同时,也受到土壤环境的影响,而定殖于梭梭根系内部的DSE真菌,它的定殖和分布是否同样受到土壤环境的影响?本研究以调查荒漠生境梭梭DSE真菌时空分布和影响因素为主要内容,探究DSE真菌定殖与土壤因子间的相互关系及变化机制,从而揭示梭梭DSE真菌在荒漠生境的定殖规律和驱动因子,为DSE真菌促进梭梭等荒漠植物的生长提供依据,进一步为荒漠化治理及植被恢复做出贡献。
1 材料与方法
1.1 样品采集
针对我国西北沙区梭梭主要分布地带,选取安西极旱荒漠国家级自然保护区(AX)和民勤连古城国家级自然保护区(MQ)为采样点(表1),分别于2019年7月、9月、12月采集植物和土壤样品。每个样地选取以梭梭为建群种的3个小样地(间距≥10km),从每个小样地随机挑选5株间距不小于100m且长势良好的梭梭植株,清除表面枯枝落叶层后,近植株根部0-30cm土层挖取土壤作为根际土壤,并于无植株生长地采集土壤作为空白对照。样品装入已编号的自封袋内冷藏运回实验室。部分样品经自然风干后过2mm筛分离根样、土样,根样保存至4℃冰箱中用于DSE真菌定殖结构观察,土样用于土壤因子分析;部分土样保存至-80℃,用于土壤微生物群落组成及代谢功能分析。
表1 安西、民勤样地环境特征
Table 1
| 样地 Sampling site | 经纬度 Longitude and latitude | 气候特征 Climatic characteristics | 海拔 Altitude (m) | 年均气温 Average annual temperature (°C) | 年均降水量 Average annual precipitation (mm/year) |
|---|---|---|---|---|---|
| 安西 Anxi | 95.77°E 40.51°N | 中亚干旱气候 Arid climate of Central Asia | 1 300 | 8.6 | 47.5 |
| 民勤 Minqin | 103.08°E 38.62°N | 温带大陆性干旱气候 Temperate continental arid climate | 1 401 | 7.8 | 113 |
1.2 DSE真菌定殖率测定
对于每株植物,随机选取30个长约1cm的根段于试管中,加入10% KOH在100℃恒温水浴下解离1h至根段透明,采用Phillips和Hayman染色法(Phillips & Hayman 1970),根段经0.5%酸性品红染色后,由乳酸甘油脱色处理后制片,镜检观察DSE真菌侵染状况及定殖结构,并计算DSE真菌菌丝、微菌核定殖率。
$定殖率(\%)=\frac{受侵染根段数}{总镜检根段数}\times 100 \%$
1.3 土壤因子测定
土壤温度(Tem)、湿度(Hum)使用土壤温湿度记录仪L99-TWS-2实地测定。配制质量比为1:2.5的土壤:水悬浮液,用精密pH计(PHS-3C)测定pH值;速效磷(AP)采用碳酸氢钠浸提-钼锑抗比色法(Bever et al. 1996),使用0.5mol/L碳酸氢钠萃取测定;碱解氮(AN)采用碱解扩散法测定;采用灼烧质量法使用马弗炉在550℃下测定土壤有机碳(SOC)(Heiri et al. 2001);用改良的Hoffmann & Teicher(1961)的比色法测定土壤脲酶(U),活性以每克土样培养3h催化尿素分解产生NH4+-N的微克数(μg)表示;碱性磷酸酶(ALP)采用改良的Tarafdar & marschner(1994)的方法测定,活性以每克土样培养1h碱性磷酸酶转化对硝基苯磷酸二钠(pNPP)的量(μg/g)表示。
1.4 土壤微生物群落结构分析
根据改良的Bossio & Scow(1998)的方法,通过测定土壤中微生物磷脂脂肪酸组分来分析土壤微生物群落结构组成。称取干重为8.0g的新鲜土样,使用23mL提取液(甲醇:磷酸缓冲液:三氯甲烷体积比为2:0.8:1)萃取油脂。萃取物通过N2浓缩后使用有机溶剂在硅胶柱(SPE-Si,500mg/6mL)上依次洗脱进行分离纯化,纯化后的脂肪酸甲基化得到甲酯化脂肪酸样品。
使用配备MIDI软件系统的气相色谱仪(美国Agilent 6890N),结合SherlockMIS 4.5系统(Sherlock Microbial Identification System)对每个样品进行磷脂脂肪酸检测分析。MIDI软件包括自动控制校准、后续样品测序、命名气相色谱等操作,特征磷脂脂肪酸分类见表2。
表2 特征磷脂脂肪酸(PLFA)分类
Table 2
| 定殖微生物 Colonial microorganisms | 磷脂脂肪酸 Phospholipid fatty acid |
|---|---|
| AM真菌 AM fungi | 16:1 ω5c |
| 革兰氏阴性细菌 Gram negative bacteria | 16:0 2OH, 16:1 ω7c, 17:1 ω8c, 17:0 cyclo ω7c, 18:1 ω7c, 19:1 ω6c, 19:0 cyclo ω7c |
| 土壤真菌 Soil fungi | 18:2 ω6c |
| 革兰氏阳性细菌 Gram positive bacteria | 12:0 anteiso, 13:0 anteiso, 14:0, 15:0 iso, 15:0 anteiso, 16:0 iso, 17:1 iso ω9c, 17:0 iso, 17:0 anteiso, 18:0 iso, 22:0 iso |
| 放线菌 Actinomycetes | 16:0 10-methyl, 18:0 10-methyl, 18:1 ω7c 10-methyl |
1.5 土壤微生物代谢功能研究
利用具有31种碳源的生态微平板Biolog EcoplatesTM(Zak et al. 1994)测定微生物群落功能多样性,此生态板碳源底物根据有机物化学官能团、微生物生理代谢途径以及生态功能,可分为碳水化合物、羧酸、多聚物类、胺类、酚酸类以及氨基酸类6大类型。
称取干重5.0g的新鲜土壤于锥形瓶中,加入45mL无菌生理盐水,以250r/min振荡30min后按1:1 000比例稀释得到菌悬液,将生态板预热至室温后,每孔加入150μL菌悬液。
将以上生态板置于28℃恒温培养箱中黑暗下连续培养,并于24、48、72、96、120、144、168、192、216、240h后用酶标仪测590nm吸光度值。
土壤微生物代谢活性可以通过平均颜色变化率(AWCD值)表示,公式为:
$A W C D=[\Sigma(C-R)] / 31$
其中,C是每个碳源所在反应孔吸光度值,R为对照孔相应吸光度值。
土壤微生物多样性指数可以通过Shannon-Wiener指数(H)、Simpson指数(D)、McIntosh指数(U)表示,公式为:
$H=-\sum ({{P}_{i}}\times ln{{P}_{i}})$
$D=1-\sum P_{i}^{2}$
$U=\sqrt{\sum n_{i}^{2}}$
其中,Pi为第i孔相对吸光值与整个生态平板相对吸光值总和的比率;ni为第i孔相对吸光值,即(C-R)值。
1.6 数据处理方法
采用Excel 2016进行数据统计并绘图;对于DSE真菌定殖、土壤因子、土壤微生物数据采用SPSS 20.0进行方差分析,对比根际土壤与空白土壤不同环境因子差异使用独立样本t检验;采用SPSS 20.0对土壤微生物群落组成及代谢功能与DSE真菌定殖进行相关性分析;采用Canoco 4.5对土壤因子与DSE真菌定殖率和定殖特征进行冗余分析,筛选影响DSE真菌定殖的关键因子;使用R Studio 1.25对不同因子对于DSE真菌定殖的影响进行方差分解。
2 结果与分析
2.1 DSE真菌定殖特征
图1
图1
不同季节、样地深色有隔内生真菌菌丝和微菌核结构 A,B:7月安西,C,D:7月民勤,E,F:9月安西,G,H:9月民勤,I,J:12月安西,K,L:12月民勤;Hy:菌丝,M:微菌核
Fig. 1
Hyphal and microsclerotial structures of dark septate endophyte in different seasons and sampling sites. A, B: Anxi in July, C, D: Minqin in July, E, F: Anxi in September, G, H: Minqin in September, I, J: Anxi in December, K, L: Minqin in December; Hy: Hyphae, M: Microsclerotia.
表3 深色有隔内生真菌定殖结构形态时空差异
Table 3
| 季节 Season | 样地 Sampling site | 菌丝隔间距 Hyphal septum interval (μm) | 菌丝直径 Hyphal diameter (μm) | 微菌核直径 Microsclerotial diameter (μm) |
|---|---|---|---|---|
| 7月 July | 安西Anxi | 42.14±2.33a | 4.83±0.39abc | 6.30±0.11c |
| 民勤Minqin | 26.90±0.49b | 3.81±0.12bc | 7.40±0.36b | |
| 9月 September | 安西Anxi | 21.83±1.00c | 5.42±0.99ab | 5.68±0.40c |
| 民勤Minqin | 18.82±1.58c | 3.45±0.09c | 8.40±0.08b | |
| 12月 December | 安西Anxi | 18.64±0.71c | 5.88±0.70a | 8.20±0.36b |
| 民勤Minqin | 22.66±0.37c | 3.28±0.19c | 10.30±0.50a | |
| 季节Season | *** | ** | *** | |
| 样地Sampling site | ** | ** | *** | |
| 季节×样地Season×sampling site | *** | |||
注:不同小写字母表示同一列比较差异显著(P<0.05);**表示在0.01水平差异显著,***表示在0.001水平差异显著. AX:安西,MQ:民勤. 下同
Note: Different lowercase letters indicate significant difference in same column (P<0.05); ** indicates significance at the 0.01 probability level, *** indicates significance at the 0.001 probability level. AX: Anxi, MQ: Minqin. The same below.
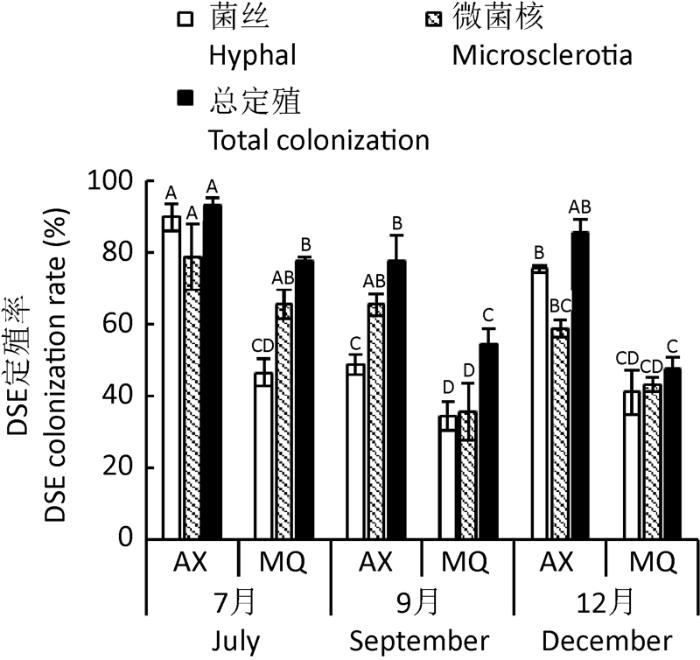
2.2 DSE真菌定殖时空分布特征
表4 季节和样地对深色有隔内生真菌定殖率影响的双因素方差分析
Table 4
| 定殖率 Colonization rate | 季节 Season | 样地 Sampling site | 季节×样地 Season×sampling site | |||||
|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | |||
| 菌丝定殖率Hyphal colonization rate | 23.209 | P<0.001 | 90.642 | P<0.001 | 7.000 | 0.010 | ||
| 微菌核定殖率Microsclerotial colonization rate | 10.158 | 0.003 | 19.242 | 0.001 | 1.363 | 0.293 | ||
| 总定殖率Total colonization rate | 14.351 | 0.001 | 57.362 | P<0.001 | 3.722 | 0.055 | ||
注:黑色加粗字体表示差异显著(P<0.05)
Note: Bold black indicates significant difference (P<0.05).
图2
图2
梭梭根系深色有隔内生真菌定殖时空分布 不同大写字母表示同种定殖率在不同季节不同样地间差异显著(P<0.05)
Fig. 2
Spatial and temporal distribution of colonization of dark septate endophyte in Haloxylon ammodendron roots. Different capital letters indicate significant difference in same colonization rate between different seasons and sampling sites (P<0.05).
2.3 土壤因子时空分布
梭梭根际土壤理化性质随季节、样地变化而改变(表5)。方差分析表明,季节、样地及其交互作用显著影响土壤湿度、pH值和碱解氮含量;季节和样地交互作用显著影响土壤脲酶活性、有机碳含量;温度和有效磷含量仅受季节显著影响,碱性磷酸酶活性仅受样地显著影响。
表5 季节和样地对梭梭根际土壤因子影响的双因素方差分析
Table 5
| 土壤因子 Soil factors | 季节 Season | 样地 Sampling site | 季节×样地 Season×sampling site | |||||
|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | |||
| 温度Temperature | 814.031 | P<0.001 | 0.381 | 0.548 | 3.05 | 0.085 | ||
| 湿度Humidity | 38.572 | P<0.001 | 20.372 | 0.001 | 19.58 | P<0.001 | ||
| pH | 30.819 | P<0.001 | 53.967 | P<0.001 | 14.694 | 0.001 | ||
| 有效磷Available phosphorus | 59.924 | P<0.001 | 3.561 | 0.084 | 1.056 | 0.378 | ||
| 碱解氮Alkali-hydrolyzale nitrogen | 4.046 | 0.045 | 87.528 | P<0.001 | 5.252 | 0.023 | ||
| 有机碳Organic carbon | 3.004 | 0.088 | 5.237 | 0.041 | 5.874 | 0.017 | ||
| 脲酶Urease | 234.453 | P<0.001 | 0.09 | 0.769 | 5.783 | 0.018 | ||
| 碱性磷酸酶Alkaline phosphatase | 814.031 | 0.264 | 10.702 | 0.007 | 1.335 | 0.3 | ||
注:黑色加粗字体表示差异显著(P<0.05)
Note: Bold black indicates significant difference (P<0.05).
土壤因子表现出明显的时空异质性(图3),除温度和有机碳外,梭梭根际土壤因子与未生长植物的空白土壤差异显著。对比不同季节发现,采自7月的土壤脲酶活性显著高于9、12月土壤,而9月的土壤有效磷含量显著高于7、12月,pH值在12月较高,有机碳在7月较高;不同样地间土壤因子表现为民勤样地的pH值显著高于安西样地,脲酶、碱性磷酸酶活性在安西样地较高,而安西样地碱解氮含量显著高于民勤样地(P<0.05)。
图3
图3
梭梭根际及空白土壤因子时空分布 不同大写字母表示同一土壤因子不同季节、不同样地间差异显著(P<0.05),*表示不同土壤(根际、空白)间差异显著
Fig. 3
Spatial and temporal distribution of soil factors in the rhizosphere and blank soils of Haloxylon ammodendron. Different capital letters indicate significant difference in same soil factor between different seasons and sampling sites (P<0.05), * indicates significant difference between the rhizosphere and blank soils.
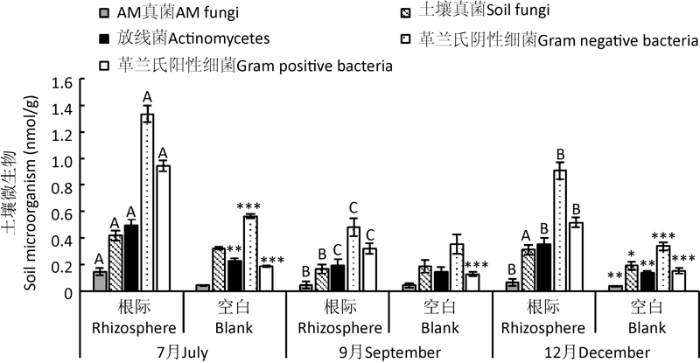
2.4 土壤微生物群落分析
土壤微生物类群在不同季节差异显著(表6)。除AM真菌外,其余4种的土壤微生物类群均受季节显著影响。
表6 季节和样地对梭梭根际土壤微生物类群影响的双因素方差分析
Table 6
| 土壤微生物类群 Soil microbial communities | 季节 Season | 样地 Sampling site | 季节×样地 Season×sampling site | |||||
|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | |||
| 土壤真菌Soil fungi | 12.475 | 0.001 | 0.049 | 0.829 | 1.592 | 0.244 | ||
| AM真菌AM fungi | 3.62 | 0.059 | 0.168 | 0.69 | 0.023 | 0.977 | ||
| 放线菌Actinomycetes | 11.325 | 0.002 | 0.637 | 0.44 | 0.696 | 0.518 | ||
| 革兰氏阴性细菌 Gram negative bacteria | 65.686 | P<0.001 | 0.002 | 0.964 | 0.128 | 0.881 | ||
| 革兰氏阳性细菌 Gram positive bacteria | 43.242 | P<0.001 | 0.425 | 0.527 | 0.123 | 0.885 | ||
注:黑色加粗字体表示差异显著(P<0.05)
Note: Bold black indicates significant difference (P<0.05).
图4
图4
不同季节梭梭根际和空白土壤微生物类群磷脂脂肪酸含量 不同大写字母表示不同季节同一种微生物差异显著(P<0.05);*表示不同土壤(根际、空白)间土壤微生物差异显著,*表示在0.05水平差异显著,**表示在0.01水平差异显著,***表示在0.001水平差异显著
Fig. 4
Phospholipid fatty acid content of soil microbial communities in the rhizosphere and blank soils of Haloxylon ammodendron in different seasons. Different capital letters indicate significant differences of the same soil microbe in different seasons (P<0.05); * indicates significance at the 0.05 probability level, ** indicates significance at the 0.01 probability level, and *** indicates significance at the 0.001 probability level.
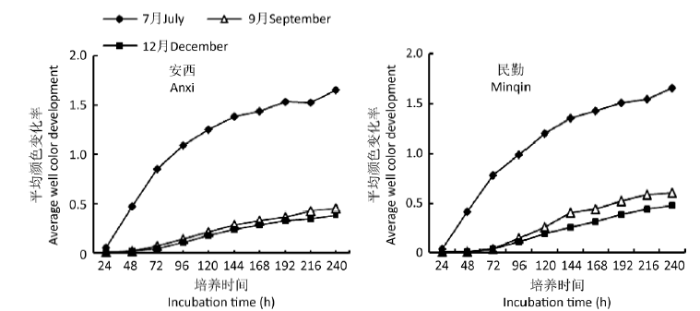
2.5 土壤微生物分解代谢功能多样性
随着培养时间的延长,安西、民勤样地土壤样品在7、9、12月AWCD值均呈s型变化,且7月AWCD值最高(图5)。各样地土壤微生物的AWCD值均于0-144h内变化速率最快,192h后趋于平缓。
图5
图5
不同样地梭梭根际土壤微生物年平均颜色变化率
Fig. 5
Changes in the average well color development (AWCD) values in the rhizosphere soil of Haloxylon ammodendron in different sampling sites.
梭梭根际土壤微生物6种碳源利用率受季节显著影响(表7)。在6大碳源中,碳水化合物利用最高,氨基酸和多聚物类次之,酚酸类和胺类的利用较低;梭梭根际土壤微生物对碳源的利用率在7月最高,且显著高于9、12月(P<0.05)。
表7 梭梭根际土壤微生物碳源的相对利用率
Table 7
| 季节 Season | 样地 Sampling site | 碳水化合物 Carbohydrate | 羧酸 Carboxylic acids | 多聚物类 Polymers | 胺类 Amine | 酚酸类 Phenolic acids | 氨基酸类 Amino acids |
|---|---|---|---|---|---|---|---|
| 7月 July | 安西Anxi | 2.90±0.18a | 1.21±0.13a | 1.01±0.05a | 0.65±0.02a | 0.5±0.01a | 1.8±0.06a |
| 民勤Minqin | 2.74±0.01a | 1.24±0.02a | 1.04±0.17a | 0.52±0.10b | 0.49±0.02a | 1.69±0.05a | |
| 9月 September | 安西Anxi | 0.59±0.05b | 0.13±0.04b | 0.55±0.05b | 0.06±0.01c | 0.03±0.01b | 0.1±0.06b |
| 民勤Minqin | 0.77±0.2b | 0.14±0.07b | 0.63±0.05b | 0.01±0.00c | 0.01±0.01b | 0.3±0.17b | |
| 12月 December | 安西Anxi | 0.53±0.09b | 0.12±0.02b | 0.38±0.07b | 0.01±0.00c | 0.05±0.02b | 0.14±0.05b |
| 民勤Minqin | 0.46±0.08b | 0.17±0.01b | 0.39±0.03b | 0.05±0.01c | 0.03±0.02b | 0.23±0.04b | |
| 季节Season | *** | *** | *** | *** | *** | *** | |
| 样地Sampling site | |||||||
| 季节×样地 Season × sampling site | |||||||
注:不同小写字母表示同一列比较差异显著(P<0.05);***表示在0.001水平差异显著
Note: Different lowercase letters indicate significant difference in same column (P<0.05); *** indicates significance at the 0.001 probability level.
梭梭根际土壤微生物多样性指数在不同季节差异显著(表8)。土壤微生物多样性指数7月最高,显著高于9、12月;同时,安西样地在7月、12月D、H、U多样性指数均高于民勤样地,而民勤样地在9月多样性指数较高,且D指数显著高于安西样地。
表8 不同季节、样地梭梭根际土壤微生物多样性指数
Table 8
| 季节 Season | 样地 Sampling site | D | H | U |
|---|---|---|---|---|
| 7月 July | 安西Anxi | 0.960±0.001a | 3.279±0.024a | 9.446±0.291a |
| 民勤Minqin | 0.960±0.001a | 3.257±0.018a | 9.399±0.303a | |
| 9月 September | 安西Anxi | 0.895±0.015c | 2.649±0.124b | 3.664±0.215bc |
| 民勤Minqin | 0.922±0.003b | 2.772±0.008b | 4.525±0.275b | |
| 12月 December | 安西Anxi | 0.925±0.008b | 2.837±0.079b | 2.836±0.227c |
| 民勤Minqin | 0.921±0.005b | 2.795±0.065b | 3.44±0.791bc |
注:不同小写字母表示同一列比较差异显著(P<0.05)
Note: Different lowercase letters indicate significant difference in same column (P<0.05).
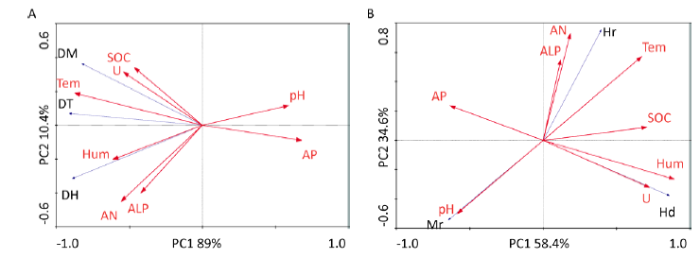
2.6 土壤因子对DSE真菌定殖的影响
冗余分析表明梭梭根际土壤因子与DSE真菌定殖率、定殖形态具有相关性(图6)。梭梭根际土壤因子对DSE真菌定殖率、定殖形态的两个轴解释量分别是97.4%、93%。
图6
图6
土壤因子与深色有隔内生真菌定殖率(A)及定殖形态的(B)冗余分析 红色箭头表示土壤因子,蓝色箭头表示深色有隔内生真菌定殖率(A)、定殖形态(B);DH:菌丝定殖率,DM:微菌核定殖率,DT:总定殖率,Hd:菌丝隔间距,Hr:菌丝直径,Mr:微菌核直径;AP:有效磷,ALP:碱性磷酸酶,SOC:有机碳,AN:碱解氮,Tem:温度,Hum:湿度,U:脲酶
Fig. 6
Redundancy analyses of soil factors and colonization rates (A) and characteristics (B) of dark septate endophyte. Red arrows indicate soil factors, blue arrows indicate colonization rates (A) and characteristics (B) of dark septate endophyte; DH: Hyphal colonization rate, DM: Microsclerotial colonization rate, DT: Total colonization rate, Hd: Hyphal septum interval, Hr: Hyphal diameter, Mr: Microsclerotial diameter; AP: Available phosphorus, ALP: Alkaline phosphatase, SOC: Organic carbon, AN: Alkali-hydrolyzed nitrogen, Tem: Temperature, Hum: Humidity, U: Urease.
梭梭根系DSE真菌定殖率与梭梭根际土壤微生物的Pearson相关性分析显示(表9),菌丝定殖率与革兰氏阴性细菌、真菌类群含量显著正相关,微菌核定殖率与AM真菌、真菌、革兰氏阳性细菌类群含量显著正相关。微菌核定殖率、总定殖率与土壤微生物6种碳源均呈显著正相关(P<0.05)。
表9 深色有隔内生真菌定殖率与土壤微生物群落和碳源代谢功能相关性分析
Table 9
| 项目 Item | 菌丝定殖率 Hyphal colonization rate | 微菌核定殖率 Microsclerotial colonization rate | 总定殖率 Total colonization rate |
|---|---|---|---|
| AM真菌AM fungi | 0.206 | 0.415* | 0.279 |
| 土壤真菌Soil fungi | 0.549* | 0.479* | 0.318 |
| 放线菌Actinomycetes | 0.350 | 0.369 | 0.224 |
| 革兰氏阴性细菌 Gram negative bacteria | 0.554* | 0.573 | 0.460 |
| 革兰氏阳性细菌 Gram positive bacteria | 0.465 | 0.577* | 0.462 |
| 碳水化合物Carbohydrate | 0.420 | 0.583* | 0.532* |
| 羧酸Carboxylic acids | 0.363 | 0.563* | 0.484* |
| 多聚物Polymers | 0.271 | 0.606** | 0.505* |
| 胺类Amine | 0.458 | 0.645* | 0.529* |
| 酚酸类Phenolic acids | 0.441 | 0.629** | 0.538* |
| 氨基酸类Amino acids | 0.406 | 0.591** | 0.486* |
注:*表示在0.05水平差异显著,**表示在0.01水平差异显著
Note: * Indicates significance at the 0.05 probability level, and ** indicates significance at the 0.01 probability level.
2.7 不同因子对DSE真菌定殖影响的方差分解
图7
图7
不同因子对梭梭深色有隔内生真菌定殖率(A)和定殖形态(B)的影响方差分解 Soil:土壤环境;Site:样地
Fig. 7
Variance partitioning of the effects of different factors on the colonization rate (A) and characteristic (B) of dark septate endophyte of Haloxylon ammodendron. Soil: Soil environment, Site: Sampling site.
3 讨论
本研究对我国西北沙区极旱荒漠调查发现,梭梭健康根系均能观察到DSE真菌存在,说明梭梭和DSE真菌能形成良好共生关系。在光学显微镜下能够观察到DSE真菌在梭梭根内形成典型的深色有隔菌丝和微菌核结构,但不同结构定殖率和定殖特征存在时空异质性。安西样地生长的梭梭根系DSE真菌定殖率较民勤样地更高,主要以菌丝为主;而民勤样地DSE主要以簇状微菌核为主。梭梭根系DSE真菌定殖率在7月最高,多细长型、微菌核较小,而12月菌丝隔间距较短,微菌核更大。同时,梭梭根际土壤环境也表现出显著的时空异质性,并与DSE真菌定殖特征表现出显著相关性。
植物根际效应对土壤生物特性至关重要(Pérez-Jaramillo et al. 2016;Santoyo et al. 2016)。研究梭梭根际生境特征发现,相比未生长植物的空白土壤,梭梭根际土壤有效磷、碱解氮含量及脲酶、碱性磷酸酶活性均更高。研究表明,植物通过根际沉积过程主动向土壤释放碳、氮等物质,影响土壤营养和酶活性,造成植物根际与无植物土壤在物理、化学等特性的差异(Bulgarelli et al. 2012;李丽娟等 2020),说明梭梭等植物对荒漠土壤具有改善作用。植物根际土壤更高的营养含量以及丰富的碳资源多样性使得土壤微生物密度、活性增强(Yang et al. 2013)。本研究中,梭梭根际土壤微生物含量高于空白土壤,与Zuo et al.(2019)研究发现蒙古沙冬青根际土壤微生物群落显著高于非根际土壤的结果一致,说明植物灌丛覆盖对荒漠土壤微生物群落活动的调控具有重要作用。此外,梭梭根际革兰氏阳性细菌含量显著高于空白土壤,这可能与革兰氏阳性细菌的膜泡能维持膜的稳定性,并能将不良物质携带出体外有关(Cao & Lin 2021),使革兰氏阳性细菌对胁迫环境具有较强的耐受性(Liao et al. 2013)。梭梭根际土壤与空白土壤的革兰氏阳性细菌等微生物含量显著的差异,预示梭梭对荒漠环境生物多样性保护具有良好的生态修复潜力。
梭梭生长环境直接影响DSE真菌的定殖情况。本研究中,梭梭根系DSE真菌定殖率在安西样地更高,且主要以菌丝为主,这与Xie et al.(2017)对其他荒漠植物花棒的研究结果一致。DSE菌丝在宿主体内的延伸扩大了根系吸收面积,促进植物对水分和养分的吸收,安西属于极旱荒漠环境,年均降雨量少,DSE菌丝的大量定殖使梭梭能更好适应极端干旱环境(贺超等 2020)。方差分解结果显示,除不同空间的样地差异对DSE真菌定殖造成显著影响外,采样时间的季节差异也是影响DSE真菌定殖的重要因素。本研究中,梭梭根系DSE真菌定殖率在7月显著高于9、12月,随季节推移,菌丝从细长型逐渐变短、微菌核直径增大,与Dasila et al.(2020)发现DSE真菌在生长季的定殖率较高,内生真菌的形态具有季节性差异的结果一致。研究表明,植物内生真菌通常会随着根系的生长而伸长,不断侵染新生根系,使内生真菌定殖受到植物生长的影响(Rayment et al. 2020),而后者又与季节变化直接相关,因此内生真菌的定殖出现季节性变化(Sommerfeld et al. 2013),这部分解释了本研究结果7月植物生长旺盛季节梭梭DSE真菌定殖率最高且菌丝较细长;而12月植物休眠季节,微菌核作为营养贮藏体直径增大,用以增强宿主植物抗逆性。
大量研究表明,植物内生真菌分布与土壤理化性质密切相关(Li et al. 2018)。方差分解结果表示,尽管生长于植物根系内部,但土壤因子同样能影响梭梭根系DSE真菌分布。相关性分析表明,梭梭根系DSE真菌分布主要与pH值、碱解氮、有效磷含量以及温、湿度显著相关。DSE真菌定殖率和菌丝直径与碱解氮含量呈正相关,而微菌核直径与碱解氮负相关。已有研究报道,土壤碱解氮能灵敏地反映土壤氮水平(赵业婷等 2013),氮素升高能够改善土壤环境、提高土壤肥力(Farrell et al. 2014),加速微生物生长代谢(Shao et al. 2019),支持了本研究中DSE真菌定殖率与碱解氮含量正相关;同时,氮含量较高的土壤,DSE菌丝较粗有利于养分吸收,而氮含量较低的土壤,微菌核较大的原因可能为了贮存养分,这与Xie et al.(2017)认为植物根系氮含量影响DSE真菌形态结果一致。梭梭DSE真菌分布与有效磷负相关,由于DSE真菌能够溶解土壤磷,促进宿主对磷的吸收(Barrow & Osuna 2002),从而影响DSE真菌定殖,与Ruotsalainen et al.(2002)研究高山草本植物发现DSE真菌在低磷环境更丰富的结果一致。在其他菌根真菌的研究中,Li et al.(2005)研究结果也表示土壤全磷和有效磷均与AM定殖负相关。土壤酸碱度在6.0-7.5时植物对养分利用率最高,酸碱度的增加会限制养分的利用率(Asghar et al. 2008)。本研究中土壤pH值在7.8-9.3之间,并与DSE真菌定殖呈负相关,推测碱性土壤可能通过限制植物对土壤养分的利用率抑制DSE真菌定殖(Xie et al. 2017)。本研究中,土壤温湿度与DSE真菌分布正相关,研究表明土壤水分下降会减弱养分在土壤中的迁移速率,使土壤微生物生物量和活性降低(Schjønning et al. 2003),而土壤温度通过改变土壤湿度和养分的有效性从而对微生物产生影响(曹艳峰等 2016),这可能部分解释土壤温湿度影响梭梭DSE真菌分布的原因。此外,温度、有效磷是影响DSE真菌定殖率的主要土壤因子,湿度和碱解氮是影响DSE真菌定殖特征的主要土壤因子。
4 结论
在本研究中,梭梭能与DSE真菌形成良好共生关系,并发挥改善荒漠土壤环境的潜力。梭梭DSE真菌分布和定殖具有时空异质性,在植物生长季节最高,并与土壤环境密切相关。土壤温、湿度以及有效磷、碱解氮含量能显著调节DSE真菌定殖率和真菌形态。本研究对荒漠生境梭梭DSE真菌时空分布和影响因素的系统性调查有助于为荒漠化治理和生态恢复提供依据。
参考文献
Microfungal endophytes in root
DOI:10.1139/b04-171 URL [本文引用: 1]
Seasonality of arbuscular mycorrhiza and dark septate endophytes in grasses
Effect of Thermomyces fungal endophytic isolated from extreme hot desert-adapted plant on heat stress tolerance of cucumber
DOI:10.1016/j.apsoil.2017.11.004 URL [本文引用: 1]
Management of treated pulp and paper mill effluent to achieve zero discharge
DOI:10.1016/j.jenvman.2007.07.004 URL [本文引用: 1]
Atypical morphology of dark septate fungal root endophytes of Bouteloua in arid southwestern USA rangelands
Native grasses of semi-arid rangelands of the southwestern USA are more extensively colonized by dark septate endophytes (DSE) than by traditional mycorrhizal fungi. Roots of dominant grasses ( Bouteloua sp.) native to arid southwestern USA rangelands were prepared and stained using stains specific for fungi (trypan blue) and for lipids (sudan IV). This revealed extensive internal colonization of physiologically active roots by atypical fungal structures that appear to function as protoplasts, without a distinguishable wall or with very thin hyaline walls that escape detection by methods staining specifically for fungal chitin. These structures were presumed to be active fungal stages that progressed to form stained or melanized septate hyphae and microsclerotia characteristic of DSE fungi within dormant roots. The most conspicuous characteristic of these fungi were the unique associations that formed within sieve elements and the accumulation of massive quantities of lipids. This interface suggests a biologically significant location for carbon transfer between the plant and fungus. The continuous intimate association with all sieve elements, cortical and epidermal cells as well as external extension on the root surface and into the soil indicates that they are systemic and considerably more prevalent than previously thought. A fungal network associated with a mucilaginous complex observed on the root surface and its potential role in root function in dry soil is discussed. It is suggested that those fungi that non-pathogenically and totally colonize plant cells be classed as systemic endophytic fungi (SEF). This would refine the broad designation of DSE fungi. The potential mutualistic benefit of SEF for native plants in arid ecosystems based on the extent of lipid accumulation and its apparent distribution is discussed.
Phosphorus solubilization and uptake by dark septate fungi in fourwing saltbush, Atriplex canescens (Pursh) Nutt
DOI:10.1006/jare.2001.0925 URL [本文引用: 1]
Host-dependent sporulation and species diversity of arbuscular mycorrhizal fungi in a mown grassland
DOI:10.2307/2261701 URL [本文引用: 1]
Functions of arbuscular mycorrhizal fungi and dark septate endophytes in ecological restoration
Dark septate endophytic fungi of native plantsalong an altitudinal gradient in the Brazilian Atlantic forest
DOI:10.1016/j.funeco.2016.01.008 URL [本文引用: 1]
Impacts of carbon and flooding on soil microbial communities, phospholipid fatty acid profiles and substrate utilization patterns
Structure and functions of the bacterial microbiota of plants
Characterization and function of membrane vesicles in Gram-positive bacteria
DOI:10.1007/s00253-021-11140-1 URL [本文引用: 1]
The spatial distribution of soil microbes around a desert shrub of Haloxylon ammodendron
Endophytes associated with Himalayan silver birch (Betula utilis D. Don) roots in relation to season and soil parameters
DOI:10.1016/j.apsoil.2020.103513 URL [本文引用: 1]
Population demographic characteristics of Haloxylon ammodendron (C.A. Mey.) Bunge ex Fenzl in Gobi Desert of Mongolia
DOI:10.22353/mjbs URL [本文引用: 1]
Seasonal variation of arbuscular mycorrhizal fungi in temperate grasslands along a wide hydrologic gradient
We studied seasonal variation in population attributes of arbuscular mycorrhizal (AM) fungi over 2 years in four sites of temperate grasslands of the Argentinean Flooding Pampas. The sites represent a wide range of soil conditions, hydrologic gradients, and floristic composition. Lotus glaber, a perennial herbaceous legume naturalised in the Flooding Pampas, was dominant at the four plant community sites. Its roots were highly colonised by AM fungi. Temporal variations in spore density, spore type, AM root colonisation, floristic composition and soil chemical characteristics occurred in each site and were different among sites. The duration of flooding had no effect on spore density but depressed AM root colonisation. Eleven different types of spores were recognized and four were identified. Two species dominated at the four sites: Glomus fasciculatum and Glomus intraradices. Spore density was highest in summer (dry season) and lowest in winter (wet season) with intermediate values in autumn and spring. Colonisation of L. glaber roots was highest in summer or spring and lowest in winter or autumn. The relative density of G. fasciculatum and G. intraradices versus Glomus sp. and Acaulospora sp. had distinctive seasonal peaks. These seasonal peaks occurred at all four sites, suggesting differences among AM fungus species with respect to the seasonality of sporulation. Spore density and AM root colonisation when measured at any one time were poorly related to each other. However, spore density was significantly correlated with root colonisation 3 months before, suggesting that high colonisation in one season precedes high sporulation in the next season.
Soil microbial organic nitrogen uptake is regulated by carbon availability
DOI:10.1016/j.soilbio.2014.07.003 URL [本文引用: 1]
Species diversity and spatial distribution of dark septate endophytic fungi in Glycyrrhiza uralensis in arid area of Northwest China
Loss on ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results
DOI:10.1023/A:1008119611481 URL [本文引用: 1]
A colorimetric technique for determining urease activity in soil
Dark septate endophytes: a review of facultative biotrophic root-colonizing fungi
DOI:10.1046/j.1469-8137.1998.00265.x
PMID:33862835
[本文引用: 2]

Dark septate root endophytes (DSE) are conidial or sterile fungi (Deuteromycotina, Fungi Imperfecti) likely to be ascomycetous and colonizing plant roots. They have been reported for nearly 600 plant species representing about 320 genera and 100 families. DSE fungi occur from the tropics to arctic and alpine habitats and comprise a heterogeneous group that functionally and ecologically overlaps with soil fungi, saprotrophic rhizoplane-inhabiting fungi, obligately and facultatively pathogenic fungi and mycorrhizal fungi. Numerous species of undescribed sterile and anamorphic taxa may also await discovery. Although DSE are abundant in washed root and soil samples from various habitats, and are easily isolated from surface-sterilized roots of ecto-, ectendo-, endo- and non-mycorrhizal host species, their ecological functions are little understood. Studies of DSE thus far have yielded inconsistent results and only poorly illustrate the role of DSE in their natural habitats. These inconsistencies are largely due to the uncertain taxonomic affinities of the strains of DSE used. In addition, because different strains of a single anamorph taxon seem to vary greatly in function, no clear generalizations on their ecological role have been drawn. This paper reviews the current literature on DSE and the ecology and discusses the need for and direction of future research.
Contrasting preferences of arbuscular mycorrhizal and dark septate fungi colonizing boreal and subarctic Avenella flexuosa
DOI:10.1007/s00572-013-0526-7
PMID:24061928
[本文引用: 1]

Arbuscular mycorrhizal (AM) and dark septate endophytic (DSE) fungi are ubiquitous in grass roots, but their colonizations may vary according to latitudinal gradient and site conditions. We investigated how vegetation zone (boreal vs. subarctic), humus thickness, and site openness affect root fungal colonizations of the grass Avenella flexuosa. More precisely, we hypothesized that AM and DSE fungal colonizations would have different responses to environmental conditions such that AM fungi could be more common in boreal zone, whereas we expected DSE fungi to be more affected by the amount of humus. We found site openness to affect AM and DSE fungi in a contrasting manner, in interaction with the vegetation zone. AM colonization was high at open coastal dunes, whereas DSE fungi were more common at forested sites, in the boreal zone. Humus thickness affected AM fungi negatively and DSE fungi positively. To conclude, the observed AM and DSE fungal colonization patterns were largely contrasting. AM fungi were favored in seashore conditions characterized by thin humus layer, whereas DSE fungi were favored in conditions of higher humus availability.
Seasonality of arbuscular mycorrhizal symbiosis and dark septate endophytes in a grassland site in Southwest China
DOI:10.1016/j.femsec.2005.04.011 URL [本文引用: 1]
Characteristics of nutrient content and enzyme activity in the rhizosphere and bulk soils of four suitable plant species in the hydro-fluctuation zone of the Three Gorges Reservoir
Distribution of fungal endophytes in roots of Stipa krylovii across six vegetation types in grassland of northern China
DOI:10.1016/j.funeco.2017.11.001 URL [本文引用: 1]
Characteristics of soil microbial community functional and structure diversity with coverage of Solidago canadensis L
DOI:10.1007/s11771-013-1544-5 URL [本文引用: 1]
Seasonal and temporal dynamics of arbuscular mycorrhizal and dark septate endophytic fungi in a tallgrass prairie ecosystem are minimally affected by nitrogen enrichment
DOI:10.1007/s00572-008-0165-6 URL [本文引用: 1]
Isolation and morphological and metabolic characterization of common endophytes in annually burned tallgrass prairie
DOI:10.3852/09-212 URL [本文引用: 1]
Arbuscular mycorrhizal associations and occurrence of dark septate endophytes in the roots of Brazilian weed plants
DOI:10.1007/s00572-013-0519-6
PMID:23912812
[本文引用: 1]

The ecology of weed plants includes their interactions with soil microorganisms, such as mutualistic partners that may contribute to their adaptation and competitive success in the agricultural fields. Despite the importance of microorganisms to plant growth, knowledge on weed-symbiont associations is still incipient compared to crops. Thus, a survey for the presence of arbuscular mycorrhiza (AM) and dark septate endophyte (DSE) associations in the roots of 50 weed species was done in three distinct areas during the dry and rainy seasons. We found that 41 and 29 out of the 50 species were associated with AM fungi and DSE, respectively, and 27 species presented both associations. All the plant species not forming AM belong to families thought to be nonmycorrhizal, such as Amaranthaceae, Commelinaceae, Brassicaceae, and Cyperaceae. The most common morphotype of AM observed was the Arum-type. No significant differences were found in root length colonization between the areas or seasons. For 19 species surveyed, this is the first report on their mycorrhizal status.
Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection
DOI:10.1016/S0007-1536(70)80110-3 URL [本文引用: 1]
Novel root fungal consortium associated with a dominant desert grass
DOI:10.1128/AEM.02769-07
PMID:18344349
[本文引用: 1]

The broad distribution and high colonization rates of plant roots by a variety of endophytic fungi suggest that these symbionts have an important role in the function of ecosystems. Semiarid and arid lands cover more than one-third of the terrestrial ecosystems on Earth. However, a limited number of studies have been conducted to characterize root-associated fungal communities in semiarid grasslands. We conducted a study of the fungal community associated with the roots of a dominant grass, Bouteloua gracilis, at the Sevilleta National Wildlife Refuge in New Mexico. Internal transcribed spacer ribosomal DNA sequences from roots collected in May 2005, October 2005, and January 2006 were amplified using fungal-specific primers, and a total of 630 sequences were obtained, 69% of which were novel (less than 97% similarity with respect to sequences in the NCBI database). B. gracilis roots were colonized by at least 10 different orders, including endophytic, coprophilous, mycorrhizal, saprophytic, and plant pathogenic fungi. A total of 51 operational taxonomic units (OTUs) were found, and diversity estimators did not show saturation. Despite the high diversity found within B. gracilis roots, the root-associated fungal community is dominated by a novel group of dark septate fungi (DSF) within the order Pleosporales. Microscopic analysis confirmed that B. gracilis roots are highly colonized by DSF. Other common orders colonizing the roots included Sordariales, Xylariales, and Agaricales. By contributing to drought tolerance and nutrient acquisition, DSF may be integral to the function of arid ecosystems.
Seasonal patterns of fungal colonisation in Australian native plants of different ages
DOI:10.1007/s13199-019-00661-z URL [本文引用: 3]
Seasonality of root fungal colonization in low-alpine herbs
Arbuscular mycorrhizal (AM) and dark septate endophytic (DSE) fungal colonization of Alchemilla glomerulans, Carex vaginata, Ranunculus acris ssp. pumilus and Trollius europaeus growing in low-alpine meadows in the Finnish subarctic were studied at different times during the growing season. Fungal colonization was correlated to soil soluble phosphorus (P) concentration. The influence of flower bud removal on fungal colonization was investigated in A. glomerulans, C. vaginata and R. acris and the correlation between AM and DSE colonization was studied. The fungal colonization patterns were found to be species-specific. R. acris maintained a relatively high rate of fungal colonization throughout the summer, while the rates of colonization of T. europaeus were lower and decreased towards the end of the season. A. glomerulans had constant arbuscular and vesicular colonization throughout the summer, but hyphal and DSE colonization declined towards the end of the season. C. vaginata did not form arbuscular mycorrhiza, but was colonized by DSE fungi and hyaline septate hyphae throughout the season. The soil soluble P concentration showed some seasonal variation, but was also highly variable between the study sites. Bud removal decreased arbuscular colonization of R. acris, but no unique effects were seen in any other parameters or the other species studied. The root fungal parameters correlated with soil P in some species at some sites, but no consistent trend was found. DSE colonization was positively correlated with root vesicular and hyphal colonization in some cases. The differences in fungal colonization parameters may be related to species-specific phenologies.
Dark septate endophyte decreases stress on rice plants
DOI:10.1016/j.bjm.2016.09.018 URL [本文引用: 1]
Plant growth-promoting bacterial endophytes
DOI:10.1016/j.micres.2015.11.008 URL [本文引用: 1]
Linking soil microbial activity to water-and air-phase contents and diffusivities
DOI:10.2136/sssaj2003.1560 URL [本文引用: 1]
Secondary successional forests undergo tightly-coupled changes in soil microbial community structure and soil organic matter
DOI:10.1016/j.soilbio.2018.10.004 URL [本文引用: 1]
High winter diversity of arbuscular mycorrhizal fungal communities in shallow and deep grassland soils
DOI:10.1016/j.soilbio.2013.06.002 URL [本文引用: 1]
Effect of desertification on productivity in a desert steppe
DOI:10.1038/srep27839 URL [本文引用: 1]
Phosphatase activity in the rhizosphere and hyphosphere of VA mycorrhizal wheat supplied with inorganic and organic phosphorus
DOI:10.1016/0038-0717(94)90288-7 URL [本文引用: 1]
Determinants of root-associated fungal communities within Asteraceae in a semi-arid grassland
DOI:10.1111/1365-2745.12197 URL [本文引用: 1]
Spatial dynamics of dark septate endophytes in the roots and rhizospheres of Hedysarum scoparium in Northwest China and the influence of edaphic variables
DOI:10.1016/j.funeco.2017.01.007 URL [本文引用: 3]
Sand-fix effects of Haloxylon ammodendron forests under the different densities and patterns under wind tunnel test
Effect of drip irrigation with brackish water on the soil chemical properties for a typical desert plant (Haloxylon ammodendron) in the Manas river basin
DOI:10.1002/ird.v69.3 URL [本文引用: 1]
Comparison of soil microbial community catabolic diversity between rhizosphere and bulk soil induced by tillage or residue retention
Induced salt tolerance of ryegrass by Bacillus subtills strain WM13-24 from the rhizosphere of Haloxylon ammodendron
DOI:10.1016/j.chnaes.2018.12.006 URL
Functional diversity of microbial communities: a quantitative approach
DOI:10.1016/0038-0717(94)90131-7 URL [本文引用: 1]
Micro-ecosystem associated with the rhizosphere of Lycium barbarum from the loess plateau and the mechanisms of symbiotic fungal inoculation on the host plant growth and drought resistance
Spatial and temporal distribution of arbuscular mycorrhiza fungi and dark septate endophytes in Hedysarum scoparium from northwest desert belt
Study on spatial variability and change of soil alkali- hydrolyzable nitrogen in Guanzhong Basin county-level farmland
Plant cover of Ammopiptanthus mongolicus and soil factors shape soil microbial community and catabolic functional diversity in the arid desert in Northwest China
DOI:10.1016/j.apsoil.2019.103389 URL [本文引用: 1]