Botryosphaeriaceous fungi comprised plant endophytes, saprobes and parasites mostly associated with woody hosts (Phillips et al. 2013; Ariyawansa et al. 2016; Dissanayake et al. 2016; Slippers et al. 2017). Botryosphaeria Ces. & de Not. was established in 1863 by Cesati & de Notaris based on twelve species including Botryosphaeria dothidea which was designated as the lecotype by Barr (1972). Earlier species of Botryosphaeria had been described mostly based on their sexual morph and host associations, which led to the introduction of a large number of species (Cesati & de Notaris 1863; de Notaris 1863; Saccardo 1877, 1882; Grossenbacher & Duggar 1911; Putterill 1919; Trotter 1928). So far, more than 200 epithets have been included in Index Fungorum (
The polyphyletic status of Botryosphaeria sensu lato has been well documented with species mostly encompassed in various genera of the Botryosphaeriaceae (Slippers et al. 2004; Crous et al. 2006; Phillips et al. 2008, 2013; Dissanayake et al. 2016). Based on the phylogenetic analysis of five loci (SSU, LSU, ITS, TUB and tef1-α), seven species was recognized within Botryosphaeria s. str. by Phillips et al. (2013), namely B. agaves (Henn.) E.J. Butler, B. corticis (Demaree & Wilcox) Arx & E. Müll., B. dothidea (Moug.) Ces. & De Not., B. fabicerciana (S.F. Chen bis, Pavlic, M.J. Wingf. & X.D. Zhou) A.J.L. Phillips & A. Alves, B. fusispora Boonmee, J.K. Liu & K.D. Hyde, B. ramosa (Pavlic, T.I. Burgess & M.J. Wingf.) A.J.L. Phillips & A. Alves and B. scharifii Abdollahz., Zare & A.J.L. Phillips. Subsequently, other eight species namely B. auasmontanum F.J.J. Van der Walt, Slippers & G.J. Marais, B. kuwatsukai (Hara) G.Y. Sun & E. Tanaka, B. minutispermatia Ariyaw., K.D. Hyde & Zi Y. Liu, B. pseudoramosa G.Q. Li & S.F. Chen, B. qingyuanensis G.Q. Li & S.F. Chen, B. rosaceae Y.P. Zhou, Y. Zhang ter., B. sinensia Y.P. Zhou, Y. Zhang ter. and B. wangensis G.Q. Li & S.F. Chen were described (Slippers et al. 2014; Xu et al. 2015; Ariyawansa et al. 2016; Zhou et al. 2016, 2017; Li et al. 2018). So far, fifteen species have been included in Botryosphaeria s. str.
Botryosphaeriacous fungi associated with blueberries have been widely documented (Kong et al. 2010; Yu et al. 2012, 2013, 2013; Xu et al. 2015; Xu 2016; Wang et al. 2016; Dou et al. 2017). Three species of Botryosphaeria have been reported from blueberries (Vaccinium spp.), namely B. corticis, B. dothidea and B. vaccinii (Arx & Müller 1954; Barr 1972; Phillips et al. 2006; Wright & Harmon 2010; Yu et al. 2012; Xu et al. 2015). In the course of an ongoing survey of blueberry diseases in China, two new taxa with general characteristics of Botryosphaeria were collected. Molecular characteristics as well as the phylogeny based on combined ITS, LSU, tef1-α and TUB nuDNA sequences support their new status within Botryosphaeria.
1 MATERIALS AND METHODS
1.1 Sampling, isolation and morphology
Cankered stems of blueberries were collected from Fujian Province, China in 26 February 2018. About 20cm cankered branches were cut for sampling. The wood segments were first surface sterilized (Pavlic et al. 2004), cut about 0.5cm×0.5cm×0.2cm from the junction of disease and health, and then incubated on malt extract agar (MEA). Isolates grown on MEA were kept at ambient temperatures (about 28°C) in the dark. Fungal isolates were deposited in Beijing Forestry University (BJFU) with duplicates in the China General Microbiological Culture Collection Center (CGMCC). Herbarium specimens were deposited in Herbarium Mycologicum Academiae Sinicae (HMAS).
To induce sporulation, colonies resembling Botryosphaeriaceae spp. were selected and transferred to synthetic nutrient-poor agar (SNA) with sterilized pine needles placed onto the medium. Plates were incubated at 28°C under continuous near-UV light for two weeks. Released conidia and squash mounts of pycnidia formed on the pine needles were mounted in water on microscope slides and examined microscopically. Measurements and digital photographs were made using a Nikon Coolpix 995 digital camera connected to a trinocular Leitz Orthoplan microscope and processed with Adobe Photoshop Elements 10 software. Measurements of conidia, paraphyses and conidiogenous cells were made from water mounts.
1.2 DNA extraction and PCR amplification
DNA was extracted from mycelium grown on MEA plates with CTAB plant genome DNA fast extraction kit (Aidlab Biotechnologies Co., Ltd, Beijing, China). The internal transcribed spacer of rDNA (ITS) was amplified and sequenced with primers ITS1 and ITS4 (White et al. 1990). The translation elongation factor-1α (tef1-α) was amplified and sequenced with primers EF1-688F and EF1-1251R (Alves et al. 2008). The β-tubulin gene (TUB) was amplified and sequenced with primers Bt2a and Bt2b (Glass & Donaldson 1995). The 28S large subunit nuDNA (LSU) was amplified and sequenced with primers LR0R and LR5 (Vilgalys & Hester 1990). PCR amplification and sequencing followed the protocol of Zhang et al. (2009).
1.3 Sequence alignment and phylogenetic analysis
The combined loci of ITS, LSU, tef1-α and TUB were used to infer the phylogenetic relationships among different species of Botryosphaeria by Maximum parsimony (MP) and MrBayes analyses. Sequences generated were analyzed with other sequences obtained from GenBank (Table 1). Alignments were conducted in MEGA v. 6 (Tamura et al. 2013) and phylogenetic analyses performed in PAUP v. 4.0b10 (Swofford 2002) and MrBayes v. 3.1.2 (Ronquist & Huelsenbeck 2003). Prior to phylogenetic analysis, ambiguous sequences at the start and the end were deleted and gaps manually adjusted to optimize the alignments. MP was used to conduct heuristic searches as implemented in PAUP with the default options method (Zhang et al. 2008). Analyses were done under different parameters of maximum parsimony criteria as outlined in Zhang et al. (2008). Clade stability was assessed in a bootstrap analysis with 500 replicates, random sequence additions with maxtrees set to 500 and other default parameters as implemented in PAUP. For the MrBayes analysis, the best-fit model of nucleotide evolution (GTR+I+G) was selected by Akaike information criterion (AIC; Posada & Buckley 2004) in MrModeltest v. 2.3. The metropolis-coupled Markov Chain Monte Carlo (MCMCMC) approach was used to calculate posterior probabilities (Huelsenbeck & Ronquist 2005). A preliminary Bayesian inference (BI) analysis using MrBayes software revealed that the Markov Chain Monte Carlo (MCMC; Huelsenbeck & Ronquist 2001) steady state was reached after less than 10 000 generations (the average standard deviation of split frequencies was constantly below 0.01). A conservative burn-in of 100 trees was chosen and a full analysis of 5 000 000 generations was carried out with sampling every 100 generations. Trees were viewed in TREEVIEW. The nucleotide sequences generated in this paper were deposited in GenBank (Table 1). Trees and alignments were deposited in TreeBase (http://purl.org/phylo/treebase/phylows/ study/TB2:S27260?x-access-code=861cea5bca6c572233daa22d34caad22&format=html).
Table 1 Isolates of Botryosphaeria species used in the phylogenetic study
| Species | Culture/Specimen No. | Host | Location | GenBank accession numbers | |||
|---|---|---|---|---|---|---|---|
| ITS | LSU | tef1-α | TUB | ||||
| Botryosphaeria agaves | CBS 133992 ex-neotype | Agave sp. | Thailand | JX646791 | JX646808 | JX646856 | JX646841 |
| B. agaves | MFLUCC 10-0051 | Agave sp. | Thailand | JX646790 | JX646807 | JX646855 | JX646840 |
| B. auasmontanum | CMW 25413 ex-type | Pinus sp. | Namibia | KF766167 | KF766332 | N/A | N/A |
| B. corticis | CBS 119047 ex-epitype | Vaccinium corymbosum | New Jersey, USA | DQ299245 | EU673244 | EU017539 | EU673107 |
| B. corticis | ATCC 22927 | Vaccinium sp. | North Carolina, USA | DQ299247 | EU673245 | EU673291 | EU673108 |
| B. dothidea | CBS 115476 ex-epitype | Prunus sp. | Crocifisso, Switzerland | AY236949 | AY928047 | AY236898 | AY236927 |
| B. dothidea | CBS 110302 | Vitis vinifera | Portugal | AY259092 | EU673243 | AY573218 | EU673106 |
| B. fabicerciana | CBS 127193 ex-type | Eucalyptus sp. | Fujian, China | HQ332197 | MF410028 | HQ332213 | KF779068 |
| B. fabicerciana | CMW 27121 | Eucalyptus sp. | Fujian, China | HQ332198 | MF410029 | HQ332214 | KF779069 |
| B. fujianensis | CGMCC 3.19099 ex-type | Vaccinium uliginosum | Fujian, China | MH491973 | MH562326 | MH491977 | MH562330 |
| B. fujianensis | BJFUCC 180226-3 | Vaccinium uliginosum | Fujian, China | MW251380 | MW251381 | MW251388 | MW251379 |
| B. fujianensis | BJFUCC 180226-4 | Vaccinium uliginosum | Fujian, China | MW251384 | MW251383 | MW251389 | MW251390 |
| B. fusispora | MFLUCC 10-0098 ex-type | Entada sp. | Thailand | JX646789 | JX646806 | JX646854 | JX646839 |
| B. fusispora | MFLUCC 11-0507 | Caryota sp. | Thailand | JX646788 | JX646805 | JX646853 | JX646838 |
| B. kuwatsukai | CBS 135219 ex-epitype | Malus domestica | Shaanxi, China | KJ433388 | N/A | KJ433410 | N/A |
| B. kuwatsukai | LSP 5 | Pyrus sp. | Shaanxi, China | KJ433395 | N/A | KJ433417 | N/A |
| B. dolichospermatii | CGMCC 3.19096 ex-type | Vaccinium uliginosum | Fujian, China | MH491970 | MH562323 | MH491974 | MH562327 |
| B. dolichospermatii | CGMCC 3.19097 | Vaccinium uliginosum | Fujian, China | MH491971 | MH562324 | MH491975 | MH562328 |
| B. dolichospermatii | CGMCC 3.19098 | Vaccinium uliginosum | Fujian, China | MH491972 | MH562325 | MH491976 | MH562329 |
| B. minutispermatia | GZCC 16-0013 ex-type | Dead wood | China | KX447675 | N/A | KX447678 | N/A |
| B. minutispermatia | GZCC 16-0014 | Dead wood | China | KX447676 | N/A | KX447679 | N/A |
| B. pseudoramosa | CERC 2001 ex-type | Eucalyptus hybrid | Guangxi, China | KX277989 | MF410031 | KX278094 | KX278198 |
| B. pseudoramosa | CERC 2983 | Melastoma sanguineum | Guangdong, China | KX277992 | MF410034 | KX278097 | KX278201 |
| B. qingyuanensis | CERC 2946 ex-type | Eucalyptus hybrid | Guangdong, China | KX278000 | MF410042 | KX278105 | KX278209 |
| B. qingyuanensis | CERC 2947 | Eucalyptus hybrid | Guangdong, China | KX278001 | MF410043 | KX278106 | KX278210 |
| B. ramosa | CBS 122069 ex-type | Eucalyptus camaldulensis | Bell Gorge, Australia | EU144055 | KF766333 | EU144070 | KF766132 |
| B. ramosa | CGMCC 3.18004 | Acacia sp. | Hainan, China | KX197073 | KX197082 | KX197093 | KX197100 |
| B. ramosa | CGMCC 3.18006 | Myrtaceae | Guangdong, China | KX197072 | KX197081 | KX197092 | KX197099 |
| B. rosaceae | CGMCC 3.18007 ex-type | Malus sp. | Shandong, China | KX197074 | KX197083 | KX197094 | KX197101 |
| B. rosaceae | CGMCC 3.18008 | Amygdalus sp. | Shandong, China | KX197075 | KX197084 | KX197095 | KX197102 |
| B. scharifii | CBS 124703 ex-type | Mangifera indica | Tehran, Iran | JQ772020 | N/A | JQ772057 | N/A |
| B. scharifii | CBS 124702 | Mangifera indica | Hormozgan, Iran | JQ772019 | N/A | JQ772056 | N/A |
| B. sinensia | CGMCC 3.17722 ex-type | Populus sp. | Henan, China | KT343255 | KX197089 | MH392264 | KY825091 |
| B. sinensia | CGMCC 3.17723 | Morus sp. | Henan, China | KT343254 | KX197090 | KU221233 | KX197107 |
| B. wangensis | CERC 2298 ex-type | Cunninghamina deodara | Henan, China | KX278002 | MF410044 | KX278107 | KX278211 |
| B. wangensis | CERC 2299 | Cunninghamina deodara | Henan, China | KX278003 | MF410045 | KX278108 | KX278212 |
| Cophinforma atrovirens | MFLUCC 11-0425 ex-type | Eucalyptus sp. | Thailand | JX646800 | JX646817 | JX646865 | JX646848 |
| C. atrovirens | MFLUCC 11-0655 | Eucalyptus sp. | Thailand | JX646801 | JX646818 | JX646866 | JX646849 |
| Macrophomina phaseolina | CBS 227.33 | Zea mays | Unknown | KF531825 | DQ377906 | KF531804 | KF531806 |
| M. phaseolina | CBS 162.25 | Eucalyptus sp. | Uganda | KF531826 | DQ377905 | KF531803 | KF531805 |
| Neofusicoccum luteum | CBS 110299 ex-type | Vitis vinifera | Portugal | AY259091 | AY928043 | AY573217 | DQ458848 |
| N. parvum | ATCC 58191 ex-type | Populus nigra | New Zealand | AY236943 | AY928045 | AY236888 | AY236917 |
| Neoscytalidium dimidiatum | CBS 145.78 ex-isotype | Homo sapiens | United Kingdom | KF531816 | DQ377922 | KF531795 | KF531796 |
| Neo. dimidiatum | CBS 499.66 | Mangifera indica | Unknown | KF531820 | DQ377925 | KF531798 | KF531800 |
| Neo. dimidiatum | CBS 251.49 | Juglans regia | USA | KF531819 | DQ377923 | KF531797 | KF531799 |
Note: Newly generated sequences are indicated in bold.
1.4 Pathogenicity test
To determine the pathogenicity of newly isolated taxa, four isolates (CGMCC 3.19099, CGMCC 3.19096, CGMCC 3.19097, CGMCC 3.19098) were used to perform the tests of Koch’s postulates. The tests were conducted in semi-shaded greenhouse with 2-yr-old Vaccinium seedlings. Stems for inoculating were surface sterilized with 75% ethanol and a 0.4cm×0.2cm bark was removed with a scalpel. For inoculation, corresponding size of mycelia from the margins of colonies grown on 2% MEA for 5d in the dark were taken and placed into the wounds with the mycelia facing the cambium. Subsequently, the branch was covered with moist sterilized cotton, and covered by masking tape. Three replicates were conducted in different seedlings for each isolate. Sterilized MEA were used as negative controls. After two weeks’ incubation, all inoculated branches (Fig. 1) were cut for data collection and photographing. Pathogenicity was determined by the length of the necrotic lesion. The fungi were re-isolated by cutting small pieces of wood from the edges of the lesions, cultivated in MEA at 28°C, morphologically studied and sequenced again for Koch’s postulates.
Fig. 1
Fig. 1
Symptom of blueberry twig after inoculation. A: Control; B: Inoculated by Botryosphaeria dolichospermatii; C: Inoculated by B. fujianensis.
2 RESULTS
2.1 Phylogeny analyses
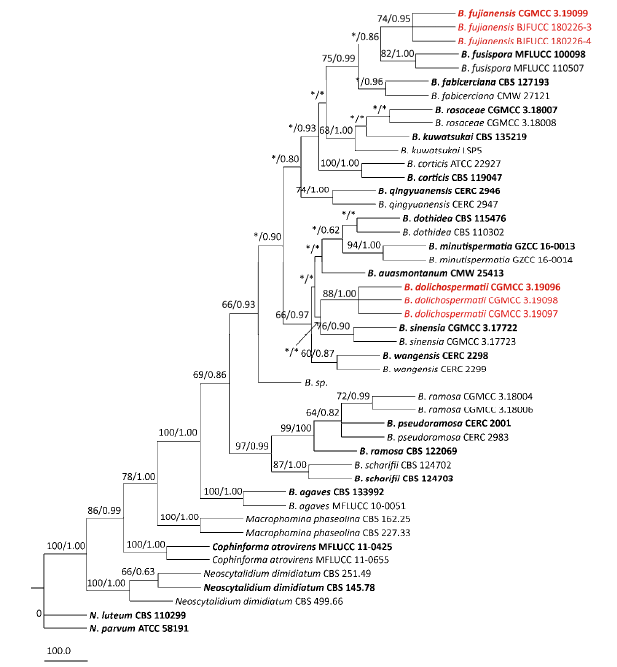
Phylogenetic analysis of the combined ITS, LSU, tef1-α and TUB sequence dataset comprising 2 268bp revealed 293 parsimony- informative characters. The outgroup taxon was Neofusicoccum luteum and N. parvum. The heuristic search with random addition of taxa (500 replicates) generated 5 000 most parsimonious trees of 511 steps (CI=0.783, RI= 0.894, RC=0.699, HI=0.217). The phylogenetic tree resulting from the Bayesian analysis using the general time reversible model of DNA evolution (Rodríguez et al. 1990), including estimation of invariable sites and assuming a discrete gamma distribution with six rate categories (GTR+Γ+G), had a topology identical to the MP tree presented. In both analyses (MP and Bayesian) the clade of Botryosphaeria received a high level of support (100 % for MP and 1.00 for BI). The subclade of B. dolichospermatii and B. fujianensis sibling to other species of Botryosphaeria, while B. dolichospermatii closely related to B. sinensia, B. auasmontanum, B. minutispermatia, B. dothidea and B. wangensis, and B. fujianensis to B. fabicerciana and B. fusispora (Fig. 2).
Fig. 2
Fig. 2
Maximum parsimony tree generated from sequence analysis of the combined ITS, tef1-α, TUB and LSU dataset of Botryosphaeria species. Designated out group taxon is Neofusicoccum luteum and N. parvum. Bootstrap support values for maximum parsimony (MP) greater than 60% are shown above at the nodes. Bayesian bootstrap (BP) posterior probability scores above 0.80 are shown under the branches (*MP value less than 60% or BP value less than 0.80). The species characterized in this study are in red, and the ex-type strains are in boldface.
2.2 Taxonomy
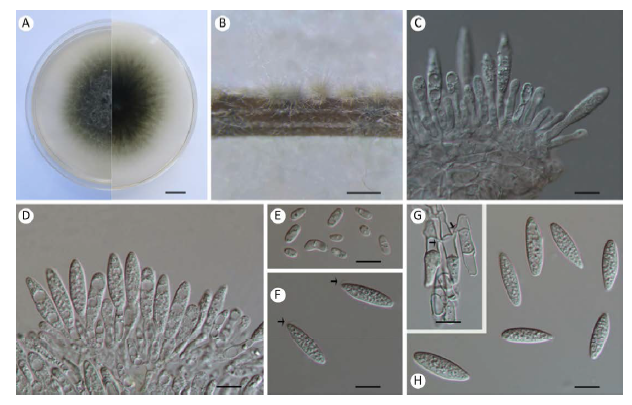
Botryosphaeria dolichospermatii Z.P. Dou, W. He, Y. Zhang, sp. nov. Fig. 3
Fig. 3
Fig. 3
Botryosphaeria dolichospermatii (From holotype, 3.19096). A: Colony growing on MEA after 5d; B: Conidiomata formed on pine needles in culture; C, D: Conidiogenous cells; E: Spermatia; F: Conidia with mucous apical sheath (arrowheads); G: Germinating conidia connected with tubes (arrowheads); H: Conidia. Scale bars: A=1cm; B=500µm; C-H=10µm.
MycoBank MB826937
Holotype: CHINA, Fujian Province, Nanping, Jianyang District, Huilong, from cankered branch of Vaccinium uliginosum, 26 February 2018, L. Zhao (HMAS 255410): ex-type living culture, CGMCC 3.19096.
Diagnosis: Botryosphaeria dolichospermatii is characterized by its conidia with mucilaginous sheath and obvious curved spermatia.
Etymology: GK. comp., dolichospermatii, referring to the relatively longer spermatia produced in culture.
Description: Sexual morph unknown. Conidiomata pycnidial, stromatic, produced on pine needles on SNA within 2-3wk, solitary, immersed to semi-immersed, iron grey to black, covered with dense mycelium, mostly uniloculate, 140-315μm diam., thick-walled, globose without papilla. Paraphyses not observed. Conidiogenous cells holoblastic, discrete, hyaline, smooth, thin-walled, cylindrical to ampulliform, producing a single conidium at the tip, (8-)9-15×(2-)3-5μm (av.= 11.5×4.1μm, n=30). Conidia fusiform, aseptate, straight, hyaline, thin-walled, smooth, apex slightly sharp, base subtruncate to bluntly rounded, sometimes with a mucous apical sheath, mostly one or two septa formed before germination, sometimes different conidia connected with tubes when germinating, (21-) 22-30(-32)×5-8μm (av. of 50 conidia=24.8× 6.5μm, L/W ratio=3.85, range from 3.14 to 4.8). Spermatia hyaline, aseptate, smooth, oblong to cylindrical, straight to obvious curved, most rounded ends, up to 9×4μm.
Culture characteristics: Colonies on MEA at 28°C in darkness oliveaceous becoming grey with reverse black. Mycelial mat moderately dense, margin irregular. Colonies reaching 53.9mm on MEA after 3d in the dark at 28°C.
Additional specimens examined: CHINA, Fujian Province, Nanping city, Jianyang district, Huilong town, from cankered branch of Vaccinium uliginosum, 26 February 2018, L. Zhao, paratype, HMAS 255411: living culture, CGMCC 3.19097; paratype, HMAS 255412: living culture, CGMCC 3.19098.
Note: Phylogenetically, Botryosphaeria dolichospermatii is distinct from but closely related to B. sinensia, B. auasmontanum, B. minutispermatia, B. dothidea and B. wangensis. Morphologically, the condia of B. dolichospermatii [(21-)22-30(-32)×5-8μm] are larger than those of B. auasmontanum [(8-)8.5-11.5(-13)× (2.5-)3-4(-5)µm] and B. minutispermatia (8-14× 3-4μm) (Slippers et al. 2014; Ariyawansa et al. 2016). The larger and sometimes curved spermatia of B. dolichospermatii (up to 9×4μm) are distinguishable from those of B. dothidea (3-6×1.5-2μm), B. sinensia (4-7× 2-3μm) and B. wangensis (3.5-4.5×1-1.5μm) (Phillips et al. 2013; Zhou et al. 2017; Li et al. 2018). Furthermore, the apical sheath presenting on some conidia of B. dolichospermatii also differs from that of other five species.
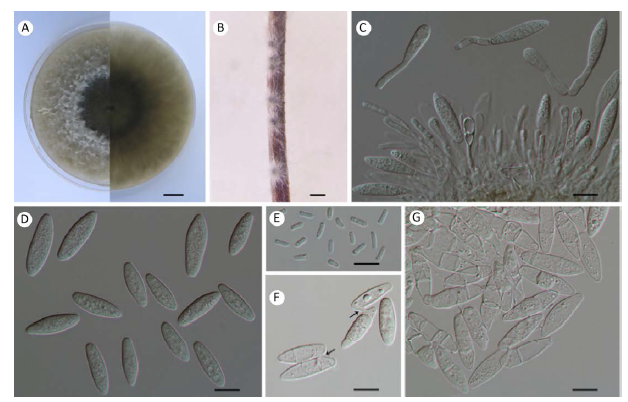
Botryosphaeria fujianensis Z.P. Dou, W. He, Y. Zhang sp. nov. Fig. 4
Fig. 4
Fig. 4
Botryosphaeria fujianensis (From holotype, 3.19099). A: Colony growing on MEA after 5d; B: Conidiomata formed on pine needles in culture; C: Conidiogenous cells and paraphyses; D: Young conidia; E: Spermatia; F: Connected conidia with tubes (arrowheads); G: Aged conidia. Scale bars: A=1cm; B=500µm; C-G=10µm.
MycoBank MB826938
Holotype: CHINA, Fujian Province, Nanping, Jianyang District, Huilong, from cankered branch of Vaccinium uliginosum, 26 February 2018, L. Zhao (HMAS 255413), ex-type living culture, CGMCC 3.19099.
Diagnosis: Botryosphaeria fujianensis is characterized by its larger conidia dimension, colonies with a circular area, lacking aerial mycelium in the middle, and different tef1-α sequence.
Etymology: in reference to the location where the species was first described.
Description: Sexual morph unknown. Conidiomata pycnidial, stromatic, produced on pine needles on SNA within 1-2wk, solitary, semi-immersed to superficial, iron grey to black, covered with dense mycelium, uniloculate, 140-330μm diam., thick-walled, globose without papilla. Paraphyses hyaline, septate, cylindrical, sometimes tapering towards the apices, arising between the conidiophores and conidiogenous cells, up to 62×4μm. Conidiophores when present hyaline, cylindrical, sometimes branches, smooth, 0-1-septate. Conidiogenous cells holoblastic, discrete, hyaline, smooth, thin-walled, cylindrical to ampulliform, producing a single conidium at the tip, 8-19× 2-4μm (av.=13.1×3.1μm, n=30). Conidia fusiform, aseptate, mostly straight, hyaline, thin-walled, smooth with granular contents, widest in the middle, both ends rounded, base sometimes truncate, mostly forming one to three septa before germination, (20-)21-29 (-31)×7-9μm (av. of 30 conidia=24×7.8μm, L/W ratio=3.08, range from 2.63 to 3.63), aged conidia hyaline, fusiform and becoming 0-3-septate, more shorter and slender, sometimes two aged conidia connected with tube, 19-24×5-7μm (av. of 30 conidia=21.3× 5.6μm, L/W ratio=3.87, range from 3.14 to 4.8). Spermatia hyaline, aseptate, smooth, rod-shaped, straight, most rounded ends, 4-8×2μm.
Culture characteristics: Colonies on MEA at 28°C in darkness oliveaceous becoming grey with reverse black. Abundant aerial mycelium reaching to the lid of Petri dishes, margin irregular with a circular black area lacking aerial mycelium in the middle. Colonies reaching 71.3mm on MEA after 3d in the dark at 28°C.
Additional specimens examined: CHINA, Fujian Province, Nanping city, Jianyang district, Huilong town, from cankered branch of Vaccinium uliginosum, 26 February 2018, L. Zhao, paratype, BJFU180226-3: living culture, BJFUCC180226-3; paratype, BJFU180226-4: living culture, BJFUCC180226-4.
Note: Phylogenetically, Botryosphaeria fujianensis is closely related but distinct from B. fabicerciana and B. fusispora. Morphologically, conidiomata of B. fujianensis (140-330μm) are larger than those of B. fusispora (140-180μm), while smaller than those of B. fabicerciana (346-470μm) (Liu et al. 2012; Phillips et al. 2013). The colonies of B. fujianensis with a circular area, lacking aerial mycelium in the middle differ from those of B. fabicerciana and B. fusispora.
2.3 Pathogenicity tests
Four isolates were inoculated on the blueberry stems. After two weeks of inoculation, a few black necrotic lesions were observed on the surface of branches while these were not seen in negative controls (Fig. 1). Koch’s postulates were fulfilled by successful re-isolation of pathogen from all the necrotic stems, and the morphological characteristics and DNA sequences of re-isolated pathogen was consistent with those of pathogen isolated from original diseased blueberry stems.
参考文献
Morphological and molecular data reveal cryptic speciation in Lasiodiplodia theobromae
Additions to Karst fungi 1: Botryosphaeria minutispermatia sp. nov., from Guizhou Province, China
Die Gattungen der amerosporen Pyrenomyceten
Some amerosporous Ascomycetes on Ericaceae and Empetraceae
Preliminary studies on the Dothideales in temperate North America
Schema di classificazione degli sferiacei italici aschigeri piu’ o meno appartenenti al genere Sphaeria nell’antico significato attribuitoglide Persoon
Phylogenetic lineages in the Botryosphaeriaceae
Botryosphaeriaceae: current status of genera and species
Lasiodiplodia chinensis sp. nov., a new holomorphic species from China
Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes
A contribution to the life-history, parasitism, and biology of Botryosphaeria ribis
MRBAYES: Bayesian inference of phylogenetic trees
Bayesian analysis of molecular evolution using MrBayes
In: Nielsen R (ed.)
First report of Neofusicoccum vitifusiforme causing blueberry blight of blueberry in China
Botryosphaeriaceae from Eucalyptus plantations and adjacent plants in China
Towards a natural classification of Botryosphaeriales
Lasiodiplodia gonubiensis sp. nov., a new Botryosphaeria anamorph from native Syzygium cordatum in South Africa
The Botryosphaeriaceae: genera and species known from culture
Resolving the phylogenetic and taxonomic status of dark-spored teleomorph genera in the Botryosphaeriaceae
Characterization and epitypification of Botryosphaeria corticis, the cause of blueberry cane canker
Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests
The general stochastic model of nucleotide substitution
MrBayes3: Bayesian phylogenetic inference under mixed models
Fungi veneti novi vel critici vel mycologiae venetae addendi, ser. VI
Combined multiple gene genealogies and phenotypic characters differentiate several species previously identified as Botryosphaeria dothidea
Diversity in the Botryosphaeriales: looking back, looking forward
Confronting the constraints of morphological taxonomy in the Botryosphaeriales
PAUP*
MEGA6: molecular evolutionary genetics analysis version 6.0
Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species
The pathogen causing Lasiodiplodia twig blight of blueberry
Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics
In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds.)
Identification of species in the Botryosphaeriaceae family causing stem blight on southern highbush blueberry in Florida
Multiple locus genealogies and phenotypic characters reappraise the causal agents of apple ring rot in China
Etiology, molecular detection and control of blueberry stem blight in China. PhD Dissertation,
Identification and distribution of Botryosphaeriaceae species associated with blueberry stem blight in China
Stem dieback of highbush blueberries caused by Neofusicoccum parvum in China
First report of stem blight of blueberry caused by Botryosphaeria dothidea in China
Identification of the pathogen causing twigs and stem dieback in blueberry
Multi-gene phylogeny and morphotaxonomy of Amniculicola lignicola: a novel freshwater fungus from France and its relationships to the Pleosporales
Towards a phylogenetic clarification of Lophiostoma / Massarina and morphologically similar genera in the Pleosporales
Botryosphaeria sinensia sp. nov. a new species from China
Botryosphaeria rosaceae sp. nov. and B. ramosa, new Botryosphaeriaceous taxa from China