A gene related to yeast HOS2 histone deacetylase affects extracellular depolymerase expression and virulence in a plant pathogenic fungus
2
2001
... 通过对氨基酸合成通路93个差异表达基因的In silico分析,发现其中部分差异表达基因在其他真菌已得到鉴定,如FOIG_05525、FOIG_05468、FOIG_09550和FOIG_075934个基因的同源基因参与调控真菌亮氨酸的合成(Liebmannet al. 2004 ;Queet al. 2020 );FOIG_12215和FOIG_09392的同源基因参与调控尖孢镰孢菌甜瓜专化型精氨酸的合成(Namikiet al. 2001 );FOIG_12832和FOIG_03926的同源基因参与调控白念珠菌甘氨酸的合成(McNeilet al. 2000 );FOIG_05323的同源基因参与调控烟曲霉支链氨基酸的合成(Oliveret al. 2012 );FOIG_02315的同源基因参与调控白念珠菌天冬氨酸的合成(Dahalet al. 2020 );FOIG_01066、FOIG_01154、FOIG_10878、FOIG_02315、FOIG_07232、FOIG_07170、FOIG_03322、FOIG_05025、FOIG_10638、FOIG_07565、FOIG_0314711个基因的同源基因参与调控真菌的生长发育或产孢(Puyeskyet al. 1997 ;Baidyaroyet al. 2001 ;Rodakiet al. 2006 ;Dinget al. 2010 ;Kuret al. 2010 ;Gerkeet al. 2012 ;Fuet al. 2013 ;Duet al. 2014 ;Fernandezet al. 2014 ;Dahalet al. 2020 ;Schaeferet al. 2020 );FOIG_00393、FOIG_01066、FOIG_12215、FOIG_05468、FOIG_05352、FOIG_05323、FOIG_06701、FOIG_06470、FOIG_09550、FOIG_05525、FOIG_09392、FOIG_07410、FOIG_07232、FOIG_05897、FOIG_03295、FOIG_05025、FOIG_15020、FOIG_07593、FOIG_0756519个基因的同源基因参与调控真菌的致病性或毒素产生(Chenget al. 1999 ;Baidyaroyet al. 2001 ;Namikiet al. 2001 ;Liebmannet al. 2004 ;Seonget al. 2005 ;Dinget al. 2010 ;Kingsbury & McCusker 2010;Oliveret al. 2012 ;Fuet al. 2013 ;Yaoet al. 2013 ;Duet al. 2014 ;Liet al. 2018 ;Guoet al. 2019 ;Queet al. 2020 ;Schaeferet al. 2020 ;Weiet al. 2020 )(表1).由此说明,氨基酸合成通路不仅与尖孢镰孢菌古巴专化型的氨基酸合成和厚垣孢子形成相关,其有可能参与调控该病菌的致病性. ...
... ;Baidyaroyet al. 2001 ;Namikiet al. 2001 ;Liebmannet al. 2004 ;Seonget al. 2005 ;Dinget al. 2010 ;Kingsbury & McCusker 2010;Oliveret al. 2012 ;Fuet al. 2013 ;Yaoet al. 2013 ;Duet al. 2014 ;Liet al. 2018 ;Guoet al. 2019 ;Queet al. 2020 ;Schaeferet al. 2020 ;Weiet al. 2020 )(表1).由此说明,氨基酸合成通路不仅与尖孢镰孢菌古巴专化型的氨基酸合成和厚垣孢子形成相关,其有可能参与调控该病菌的致病性. ...
Candida species rewired hyphae developmental programs for chlamydospore formation
1
2016
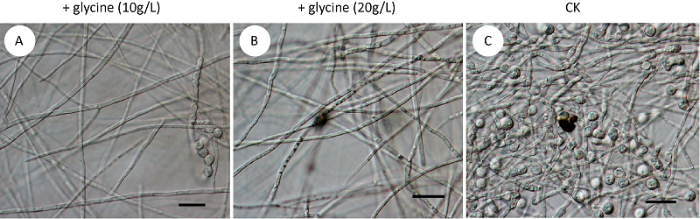
... 氨基酸如何影响真菌产孢、生长或者抑制尖孢镰孢菌古巴专化型厚垣孢子形成呢?真菌为适应不同或者变化的环境,会尽可能广泛地选择营养来源.有些真菌种类利用氨基酸的降解产物作为唯一氮源,有的则利用其作为唯一碳源,有的则两者兼有之(Rzadet al. 2018 ),如白念珠菌、新型隐球菌Cryptococcus neoformans和烟曲霉利用赖氨酸作为唯一氮源,酿酒酵母则利用赖氨酸作为唯一的碳源而非唯一氮源(Kinzelet al. 1983 ).已有报道表明,碳源的浓度与真菌厚垣孢子的形成相关,高浓度的葡萄糖抑制白色念珠菌和镰孢菌厚垣孢子的形成,缺乏或者低浓度的葡萄糖可促使镰孢菌厚垣孢子的形成(Qureshi & Page 1970;Griffin 1976;Mandal & Chaudhuri 2013;Bottcheret al. 2016 ).前期的研究发现,作为碳源的N-乙酰葡糖胺浓度达到20g/L时,尖孢镰孢菌古巴专化型的菌丝不产生厚垣孢子;当N-乙酰葡糖胺缺乏时,其菌丝可产生大量厚垣孢子(丁兆建等 2019a).由此推测,外源甘氨酸的添加导致碳源的浓度升高,从而抑制尖孢镰孢菌古巴专化型厚垣孢子的诱导形成. ...
Identification of gene clusters associated with fusaric acid, fusarin, and perithecial pigment production inFusarium verticillioides
1
2012
... 尖孢镰孢菌古巴专化型的致病因子主要包括细胞壁降解酶、信号转导通路、毒素、sRNAs和致病相关基因.在病原菌和寄主互作过程中,病原菌通过分泌一系列细胞壁降解酶降解寄主植物的细胞壁,进而将多糖等营养物质从植物细胞内释放供病原菌生长,促进病原菌的侵入和定殖;多聚半乳糖醛酸酶已被鉴定为尖孢镰孢菌古巴专化型4号生理小种的致病因子(董章勇和王振中 2012).已有报道表明,FoSlt2 MAPK信号转导通路调控尖孢镰孢菌古巴专化型的生理特性和致病性(Dinget al. 2015 ;Dinget al. 2018a ;丁兆建等 2019b;Dinget al. 2020 );cAMP-PKA信号通路G蛋白亚基FGA2和FGB1参与调控尖孢镰孢菌古巴专化型的生长发育、产孢、耐热性和致病性(Guoet al. 2016 ).镰孢菌酸(fusaric acid,FA)是镰孢菌产生的非寄主专化性毒素,对动物和人类具有中低毒性,但对植物有较高的毒性,可引起多种作物的萎蔫病和根腐病害(Brownet al. 2012 ;Niehauset al. 2014 ).国内外已报道尖孢镰孢菌古巴专化型的多个致萎相关毒素,包括FA、白僵菌素和伏马菌素B1(许文耀等 2004;陈石等 2011;李春雨等 2011;Ding et al. 2018b ;Portal et al. 2018 ).关于植物病原真菌致病相关sRNAs报道较多,灰霉菌Botrytis cinerea、大丽轮枝菌Verticillium dahlia、小麦柄锈菌Puccinia striiformis f. sp. tritici和卵菌Oomycetes产生的sRNAs效应子可跨界传输到寄主细胞中,通过沉默寄主相关免疫基因,抑制寄主植物的免疫反应(Weiberget al. 2013 ;Wanget al. 2016 ;Wanget al. 2017 );关于尖孢镰孢菌古巴专化型sRNAs的研究处于起步阶段,李敏慧等(2019)发现用该病菌接种香蕉苗根部24h后,病原菌编码Dicer 和Argonaute的基因表达显著升高,sRNA测序结果也表明,大量milRNA显著高表达.也有多个相关致病基因得到鉴定,如活性氧迸发转录因子Foatf1(Qiet al. 2013 )、编码α-1,6-甘露糖转移酶的基因FoOCH1(Liet al. 2014 )、微管蛋白β1和β2(刘远征等 2018a,2018b)和Ⅴ型肌球蛋白Myosin5的协作蛋白smy1(何壮等 2019).尽管该菌的致病机理研究已取得了显著进展,但其致病机制仍不清晰,尤其对作为初侵染源的厚垣孢子的研究也尚未引起足够的重视. ...
Research advances on pathogenic mechanism of Fusarium oxysporum
0
2011
MoLys2 is necessary for growth, conidiogenesis, lysine biosynthesis, and pathogenicity in Magnaporthe oryzae
2
2014
... 氨基酸是重要的有机化合物,其在蛋白质合成、细胞生长、发育和能量产生等多个生物学过程中起着重要作用.已有研究证明氨基酸代谢与真菌的产孢、生长和致病性相关.在人类致病菌中,赖氨酸合成基因lysF调控烟曲霉Aspergillus fumigatus的致病性(Liebmannet al. 2004 );3个精氨酸合成相关基因Car1、Agt1和Gbu1调控白念珠菌Candida albicans的生长和致病性(Schaeferet al. 2020 );天冬氨酸合成基因asd的缺失抑制白念珠菌的生长(Dahalet al. 2020 ).L-精氨酸是生防菌盾壳霉Coniothyrium minitans产孢所必需的,其衍生物一氧化氮可能介导其产孢功能(Gonget al. 2007 ).在植物病原菌中,尖孢镰孢菌甜瓜专化型F.oxysporum f. sp. melonis精氨酸合成基因ARG1缺失突变体(Namikiet al. 2001 )和灰霉菌精氨酸合成基因bcass1缺失突变体(Patelet al. 2010 )的致病性均减弱;甲硫氨酸合成基因FgMETB调控禾谷镰孢菌的孢子萌发、菌丝生长、DON毒素产生和致病性(Fuet al. 2013 );谷氨酸合成基因P5Cdh调控栗疫病菌Cryphonectria parasitica的致病性和产孢(Yaoet al. 2013 );甲硫氨酸合成基因MET13(Yanet al. 2013 )、赖氨酸合成基因MoLys2(Chenet al. 2014 )、苏氨酸合成基因MoIlv1(Duet al. 2014 )、精氨酸合成基因(MoARG1、MoARG5、MoARG6 和 MoARG7)(Zhanget al. 2015 )、亮氨酸合成基因(MoLEU1、MoLEU2、MoLEU4、LEU1、LEU2A和LEU4)(Queet al. 2020 ;Weiet al. 2020 )与稻瘟菌的生理特性和致病性密切相关.迄今,尚未有氨基酸与尖孢镰孢菌古巴专化型产孢、生长发育和致病性的相关报道. ...
... 氨基酸合成通路通过调控氨基酸的合成,影响尖孢镰孢菌古巴专化型厚垣孢子的形成.前人的研究表明,氨基酸合成基因的敲除能导致氨基酸的合成缺失,同时影响真菌生长或产孢,如白念珠菌精氨酸合成基因 Car1、Agt1和Gbu1(Schaeferet al. 2020 )及天冬氨酸合成基因asd(Dahalet al. 2020 ),禾谷镰孢菌甲硫氨酸合成基因FgMETB(Fuet al. 2013 )、栗疫病菌谷氨酸合成基因 P5Cdh(Yaoet al. 2013 )、稻瘟菌甲硫氨酸合成基因MET13(Yanet al. 2013 )、赖氨酸合成基因MoLys2(Chenet al. 2014 )、苏氨酸合成基因MoIlv1(Duet al. 2014 )、精氨酸合成基因(MoARG1、MoARG5、MoARG6和MoARG7)(Zhanget al. 2015 )、亮氨酸合成基因(MoLEU1、MoLEU2、MoLEU4、LEU1、LEU2A 和LEU4)(Queet al. 2020 ;Weiet al. 2020 ).值得指出的是,这些氨基酸合成基因的同源基因的表达水平在尖孢镰孢菌古巴专化型厚垣孢子形成过程中均发生了变化.此外,本研究发现外源甘氨酸抑制尖孢镰孢菌古巴专化型厚垣孢子的诱导形成.这些数据确认了氨基酸合成基因与尖孢镰孢菌古巴专化型厚垣孢子形成存在联系,氨基酸合成通路可通过调控氨基酸合成基因的转录,进而影响尖孢镰孢菌古巴专化型厚垣孢子的形成. ...
A putative branched-chain-amino-acid transaminase gene required for HC-toxin biosynthesis and pathogenicity inCochliobolus carbonum
1
1999
... 通过对氨基酸合成通路93个差异表达基因的In silico分析,发现其中部分差异表达基因在其他真菌已得到鉴定,如FOIG_05525、FOIG_05468、FOIG_09550和FOIG_075934个基因的同源基因参与调控真菌亮氨酸的合成(Liebmannet al. 2004 ;Queet al. 2020 );FOIG_12215和FOIG_09392的同源基因参与调控尖孢镰孢菌甜瓜专化型精氨酸的合成(Namikiet al. 2001 );FOIG_12832和FOIG_03926的同源基因参与调控白念珠菌甘氨酸的合成(McNeilet al. 2000 );FOIG_05323的同源基因参与调控烟曲霉支链氨基酸的合成(Oliveret al. 2012 );FOIG_02315的同源基因参与调控白念珠菌天冬氨酸的合成(Dahalet al. 2020 );FOIG_01066、FOIG_01154、FOIG_10878、FOIG_02315、FOIG_07232、FOIG_07170、FOIG_03322、FOIG_05025、FOIG_10638、FOIG_07565、FOIG_0314711个基因的同源基因参与调控真菌的生长发育或产孢(Puyeskyet al. 1997 ;Baidyaroyet al. 2001 ;Rodakiet al. 2006 ;Dinget al. 2010 ;Kuret al. 2010 ;Gerkeet al. 2012 ;Fuet al. 2013 ;Duet al. 2014 ;Fernandezet al. 2014 ;Dahalet al. 2020 ;Schaeferet al. 2020 );FOIG_00393、FOIG_01066、FOIG_12215、FOIG_05468、FOIG_05352、FOIG_05323、FOIG_06701、FOIG_06470、FOIG_09550、FOIG_05525、FOIG_09392、FOIG_07410、FOIG_07232、FOIG_05897、FOIG_03295、FOIG_05025、FOIG_15020、FOIG_07593、FOIG_0756519个基因的同源基因参与调控真菌的致病性或毒素产生(Chenget al. 1999 ;Baidyaroyet al. 2001 ;Namikiet al. 2001 ;Liebmannet al. 2004 ;Seonget al. 2005 ;Dinget al. 2010 ;Kingsbury & McCusker 2010;Oliveret al. 2012 ;Fuet al. 2013 ;Yaoet al. 2013 ;Duet al. 2014 ;Liet al. 2018 ;Guoet al. 2019 ;Queet al. 2020 ;Schaeferet al. 2020 ;Weiet al. 2020 )(表1).由此说明,氨基酸合成通路不仅与尖孢镰孢菌古巴专化型的氨基酸合成和厚垣孢子形成相关,其有可能参与调控该病菌的致病性. ...
Aspartate semialdehyde dehydrogenase inhibition suppresses the growth of the pathogenic fungus Candida albicans
4
2020
... 氨基酸是重要的有机化合物,其在蛋白质合成、细胞生长、发育和能量产生等多个生物学过程中起着重要作用.已有研究证明氨基酸代谢与真菌的产孢、生长和致病性相关.在人类致病菌中,赖氨酸合成基因lysF调控烟曲霉Aspergillus fumigatus的致病性(Liebmannet al. 2004 );3个精氨酸合成相关基因Car1、Agt1和Gbu1调控白念珠菌Candida albicans的生长和致病性(Schaeferet al. 2020 );天冬氨酸合成基因asd的缺失抑制白念珠菌的生长(Dahalet al. 2020 ).L-精氨酸是生防菌盾壳霉Coniothyrium minitans产孢所必需的,其衍生物一氧化氮可能介导其产孢功能(Gonget al. 2007 ).在植物病原菌中,尖孢镰孢菌甜瓜专化型F.oxysporum f. sp. melonis精氨酸合成基因ARG1缺失突变体(Namikiet al. 2001 )和灰霉菌精氨酸合成基因bcass1缺失突变体(Patelet al. 2010 )的致病性均减弱;甲硫氨酸合成基因FgMETB调控禾谷镰孢菌的孢子萌发、菌丝生长、DON毒素产生和致病性(Fuet al. 2013 );谷氨酸合成基因P5Cdh调控栗疫病菌Cryphonectria parasitica的致病性和产孢(Yaoet al. 2013 );甲硫氨酸合成基因MET13(Yanet al. 2013 )、赖氨酸合成基因MoLys2(Chenet al. 2014 )、苏氨酸合成基因MoIlv1(Duet al. 2014 )、精氨酸合成基因(MoARG1、MoARG5、MoARG6 和 MoARG7)(Zhanget al. 2015 )、亮氨酸合成基因(MoLEU1、MoLEU2、MoLEU4、LEU1、LEU2A和LEU4)(Queet al. 2020 ;Weiet al. 2020 )与稻瘟菌的生理特性和致病性密切相关.迄今,尚未有氨基酸与尖孢镰孢菌古巴专化型产孢、生长发育和致病性的相关报道. ...
... 通过对氨基酸合成通路93个差异表达基因的In silico分析,发现其中部分差异表达基因在其他真菌已得到鉴定,如FOIG_05525、FOIG_05468、FOIG_09550和FOIG_075934个基因的同源基因参与调控真菌亮氨酸的合成(Liebmannet al. 2004 ;Queet al. 2020 );FOIG_12215和FOIG_09392的同源基因参与调控尖孢镰孢菌甜瓜专化型精氨酸的合成(Namikiet al. 2001 );FOIG_12832和FOIG_03926的同源基因参与调控白念珠菌甘氨酸的合成(McNeilet al. 2000 );FOIG_05323的同源基因参与调控烟曲霉支链氨基酸的合成(Oliveret al. 2012 );FOIG_02315的同源基因参与调控白念珠菌天冬氨酸的合成(Dahalet al. 2020 );FOIG_01066、FOIG_01154、FOIG_10878、FOIG_02315、FOIG_07232、FOIG_07170、FOIG_03322、FOIG_05025、FOIG_10638、FOIG_07565、FOIG_0314711个基因的同源基因参与调控真菌的生长发育或产孢(Puyeskyet al. 1997 ;Baidyaroyet al. 2001 ;Rodakiet al. 2006 ;Dinget al. 2010 ;Kuret al. 2010 ;Gerkeet al. 2012 ;Fuet al. 2013 ;Duet al. 2014 ;Fernandezet al. 2014 ;Dahalet al. 2020 ;Schaeferet al. 2020 );FOIG_00393、FOIG_01066、FOIG_12215、FOIG_05468、FOIG_05352、FOIG_05323、FOIG_06701、FOIG_06470、FOIG_09550、FOIG_05525、FOIG_09392、FOIG_07410、FOIG_07232、FOIG_05897、FOIG_03295、FOIG_05025、FOIG_15020、FOIG_07593、FOIG_0756519个基因的同源基因参与调控真菌的致病性或毒素产生(Chenget al. 1999 ;Baidyaroyet al. 2001 ;Namikiet al. 2001 ;Liebmannet al. 2004 ;Seonget al. 2005 ;Dinget al. 2010 ;Kingsbury & McCusker 2010;Oliveret al. 2012 ;Fuet al. 2013 ;Yaoet al. 2013 ;Duet al. 2014 ;Liet al. 2018 ;Guoet al. 2019 ;Queet al. 2020 ;Schaeferet al. 2020 ;Weiet al. 2020 )(表1).由此说明,氨基酸合成通路不仅与尖孢镰孢菌古巴专化型的氨基酸合成和厚垣孢子形成相关,其有可能参与调控该病菌的致病性. ...
... ;Dahalet al. 2020 ;Schaeferet al. 2020 );FOIG_00393、FOIG_01066、FOIG_12215、FOIG_05468、FOIG_05352、FOIG_05323、FOIG_06701、FOIG_06470、FOIG_09550、FOIG_05525、FOIG_09392、FOIG_07410、FOIG_07232、FOIG_05897、FOIG_03295、FOIG_05025、FOIG_15020、FOIG_07593、FOIG_0756519个基因的同源基因参与调控真菌的致病性或毒素产生(Chenget al. 1999 ;Baidyaroyet al. 2001 ;Namikiet al. 2001 ;Liebmannet al. 2004 ;Seonget al. 2005 ;Dinget al. 2010 ;Kingsbury & McCusker 2010;Oliveret al. 2012 ;Fuet al. 2013 ;Yaoet al. 2013 ;Duet al. 2014 ;Liet al. 2018 ;Guoet al. 2019 ;Queet al. 2020 ;Schaeferet al. 2020 ;Weiet al. 2020 )(表1).由此说明,氨基酸合成通路不仅与尖孢镰孢菌古巴专化型的氨基酸合成和厚垣孢子形成相关,其有可能参与调控该病菌的致病性. ...
... 氨基酸合成通路通过调控氨基酸的合成,影响尖孢镰孢菌古巴专化型厚垣孢子的形成.前人的研究表明,氨基酸合成基因的敲除能导致氨基酸的合成缺失,同时影响真菌生长或产孢,如白念珠菌精氨酸合成基因 Car1、Agt1和Gbu1(Schaeferet al. 2020 )及天冬氨酸合成基因asd(Dahalet al. 2020 ),禾谷镰孢菌甲硫氨酸合成基因FgMETB(Fuet al. 2013 )、栗疫病菌谷氨酸合成基因 P5Cdh(Yaoet al. 2013 )、稻瘟菌甲硫氨酸合成基因MET13(Yanet al. 2013 )、赖氨酸合成基因MoLys2(Chenet al. 2014 )、苏氨酸合成基因MoIlv1(Duet al. 2014 )、精氨酸合成基因(MoARG1、MoARG5、MoARG6和MoARG7)(Zhanget al. 2015 )、亮氨酸合成基因(MoLEU1、MoLEU2、MoLEU4、LEU1、LEU2A 和LEU4)(Queet al. 2020 ;Weiet al. 2020 ).值得指出的是,这些氨基酸合成基因的同源基因的表达水平在尖孢镰孢菌古巴专化型厚垣孢子形成过程中均发生了变化.此外,本研究发现外源甘氨酸抑制尖孢镰孢菌古巴专化型厚垣孢子的诱导形成.这些数据确认了氨基酸合成基因与尖孢镰孢菌古巴专化型厚垣孢子形成存在联系,氨基酸合成通路可通过调控氨基酸合成基因的转录,进而影响尖孢镰孢菌古巴专化型厚垣孢子的形成. ...
The tig1 histone deacetylase complex regulates infectious growth in the rice blast fungusMagnaporthe oryzae
2
2010
... 通过对氨基酸合成通路93个差异表达基因的In silico分析,发现其中部分差异表达基因在其他真菌已得到鉴定,如FOIG_05525、FOIG_05468、FOIG_09550和FOIG_075934个基因的同源基因参与调控真菌亮氨酸的合成(Liebmannet al. 2004 ;Queet al. 2020 );FOIG_12215和FOIG_09392的同源基因参与调控尖孢镰孢菌甜瓜专化型精氨酸的合成(Namikiet al. 2001 );FOIG_12832和FOIG_03926的同源基因参与调控白念珠菌甘氨酸的合成(McNeilet al. 2000 );FOIG_05323的同源基因参与调控烟曲霉支链氨基酸的合成(Oliveret al. 2012 );FOIG_02315的同源基因参与调控白念珠菌天冬氨酸的合成(Dahalet al. 2020 );FOIG_01066、FOIG_01154、FOIG_10878、FOIG_02315、FOIG_07232、FOIG_07170、FOIG_03322、FOIG_05025、FOIG_10638、FOIG_07565、FOIG_0314711个基因的同源基因参与调控真菌的生长发育或产孢(Puyeskyet al. 1997 ;Baidyaroyet al. 2001 ;Rodakiet al. 2006 ;Dinget al. 2010 ;Kuret al. 2010 ;Gerkeet al. 2012 ;Fuet al. 2013 ;Duet al. 2014 ;Fernandezet al. 2014 ;Dahalet al. 2020 ;Schaeferet al. 2020 );FOIG_00393、FOIG_01066、FOIG_12215、FOIG_05468、FOIG_05352、FOIG_05323、FOIG_06701、FOIG_06470、FOIG_09550、FOIG_05525、FOIG_09392、FOIG_07410、FOIG_07232、FOIG_05897、FOIG_03295、FOIG_05025、FOIG_15020、FOIG_07593、FOIG_0756519个基因的同源基因参与调控真菌的致病性或毒素产生(Chenget al. 1999 ;Baidyaroyet al. 2001 ;Namikiet al. 2001 ;Liebmannet al. 2004 ;Seonget al. 2005 ;Dinget al. 2010 ;Kingsbury & McCusker 2010;Oliveret al. 2012 ;Fuet al. 2013 ;Yaoet al. 2013 ;Duet al. 2014 ;Liet al. 2018 ;Guoet al. 2019 ;Queet al. 2020 ;Schaeferet al. 2020 ;Weiet al. 2020 )(表1).由此说明,氨基酸合成通路不仅与尖孢镰孢菌古巴专化型的氨基酸合成和厚垣孢子形成相关,其有可能参与调控该病菌的致病性. ...
... ;Dinget al. 2010 ;Kingsbury & McCusker 2010;Oliveret al. 2012 ;Fuet al. 2013 ;Yaoet al. 2013 ;Duet al. 2014 ;Liet al. 2018 ;Guoet al. 2019 ;Queet al. 2020 ;Schaeferet al. 2020 ;Weiet al. 2020 )(表1).由此说明,氨基酸合成通路不仅与尖孢镰孢菌古巴专化型的氨基酸合成和厚垣孢子形成相关,其有可能参与调控该病菌的致病性. ...
Mitogen-activated protein kinases are associated with the regulation of physiological traits and virulence in Fusarium oxysporum f. sp. cubense
1
2015
... 尖孢镰孢菌古巴专化型的致病因子主要包括细胞壁降解酶、信号转导通路、毒素、sRNAs和致病相关基因.在病原菌和寄主互作过程中,病原菌通过分泌一系列细胞壁降解酶降解寄主植物的细胞壁,进而将多糖等营养物质从植物细胞内释放供病原菌生长,促进病原菌的侵入和定殖;多聚半乳糖醛酸酶已被鉴定为尖孢镰孢菌古巴专化型4号生理小种的致病因子(董章勇和王振中 2012).已有报道表明,FoSlt2 MAPK信号转导通路调控尖孢镰孢菌古巴专化型的生理特性和致病性(Dinget al. 2015 ;Dinget al. 2018a ;丁兆建等 2019b;Dinget al. 2020 );cAMP-PKA信号通路G蛋白亚基FGA2和FGB1参与调控尖孢镰孢菌古巴专化型的生长发育、产孢、耐热性和致病性(Guoet al. 2016 ).镰孢菌酸(fusaric acid,FA)是镰孢菌产生的非寄主专化性毒素,对动物和人类具有中低毒性,但对植物有较高的毒性,可引起多种作物的萎蔫病和根腐病害(Brownet al. 2012 ;Niehauset al. 2014 ).国内外已报道尖孢镰孢菌古巴专化型的多个致萎相关毒素,包括FA、白僵菌素和伏马菌素B1(许文耀等 2004;陈石等 2011;李春雨等 2011;Ding et al. 2018b ;Portal et al. 2018 ).关于植物病原真菌致病相关sRNAs报道较多,灰霉菌Botrytis cinerea、大丽轮枝菌Verticillium dahlia、小麦柄锈菌Puccinia striiformis f. sp. tritici和卵菌Oomycetes产生的sRNAs效应子可跨界传输到寄主细胞中,通过沉默寄主相关免疫基因,抑制寄主植物的免疫反应(Weiberget al. 2013 ;Wanget al. 2016 ;Wanget al. 2017 );关于尖孢镰孢菌古巴专化型sRNAs的研究处于起步阶段,李敏慧等(2019)发现用该病菌接种香蕉苗根部24h后,病原菌编码Dicer 和Argonaute的基因表达显著升高,sRNA测序结果也表明,大量milRNA显著高表达.也有多个相关致病基因得到鉴定,如活性氧迸发转录因子Foatf1(Qiet al. 2013 )、编码α-1,6-甘露糖转移酶的基因FoOCH1(Liet al. 2014 )、微管蛋白β1和β2(刘远征等 2018a,2018b)和Ⅴ型肌球蛋白Myosin5的协作蛋白smy1(何壮等 2019).尽管该菌的致病机理研究已取得了显著进展,但其致病机制仍不清晰,尤其对作为初侵染源的厚垣孢子的研究也尚未引起足够的重视. ...
A transcription factor FoSwi6 regulates physiology traits and virulence in Fusarium oxysporum f. sp. cubense
1
2018a
... 尖孢镰孢菌古巴专化型的致病因子主要包括细胞壁降解酶、信号转导通路、毒素、sRNAs和致病相关基因.在病原菌和寄主互作过程中,病原菌通过分泌一系列细胞壁降解酶降解寄主植物的细胞壁,进而将多糖等营养物质从植物细胞内释放供病原菌生长,促进病原菌的侵入和定殖;多聚半乳糖醛酸酶已被鉴定为尖孢镰孢菌古巴专化型4号生理小种的致病因子(董章勇和王振中 2012).已有报道表明,FoSlt2 MAPK信号转导通路调控尖孢镰孢菌古巴专化型的生理特性和致病性(Dinget al. 2015 ;Dinget al. 2018a ;丁兆建等 2019b;Dinget al. 2020 );cAMP-PKA信号通路G蛋白亚基FGA2和FGB1参与调控尖孢镰孢菌古巴专化型的生长发育、产孢、耐热性和致病性(Guoet al. 2016 ).镰孢菌酸(fusaric acid,FA)是镰孢菌产生的非寄主专化性毒素,对动物和人类具有中低毒性,但对植物有较高的毒性,可引起多种作物的萎蔫病和根腐病害(Brownet al. 2012 ;Niehauset al. 2014 ).国内外已报道尖孢镰孢菌古巴专化型的多个致萎相关毒素,包括FA、白僵菌素和伏马菌素B1(许文耀等 2004;陈石等 2011;李春雨等 2011;Ding et al. 2018b ;Portal et al. 2018 ).关于植物病原真菌致病相关sRNAs报道较多,灰霉菌Botrytis cinerea、大丽轮枝菌Verticillium dahlia、小麦柄锈菌Puccinia striiformis f. sp. tritici和卵菌Oomycetes产生的sRNAs效应子可跨界传输到寄主细胞中,通过沉默寄主相关免疫基因,抑制寄主植物的免疫反应(Weiberget al. 2013 ;Wanget al. 2016 ;Wanget al. 2017 );关于尖孢镰孢菌古巴专化型sRNAs的研究处于起步阶段,李敏慧等(2019)发现用该病菌接种香蕉苗根部24h后,病原菌编码Dicer 和Argonaute的基因表达显著升高,sRNA测序结果也表明,大量milRNA显著高表达.也有多个相关致病基因得到鉴定,如活性氧迸发转录因子Foatf1(Qiet al. 2013 )、编码α-1,6-甘露糖转移酶的基因FoOCH1(Liet al. 2014 )、微管蛋白β1和β2(刘远征等 2018a,2018b)和Ⅴ型肌球蛋白Myosin5的协作蛋白smy1(何壮等 2019).尽管该菌的致病机理研究已取得了显著进展,但其致病机制仍不清晰,尤其对作为初侵染源的厚垣孢子的研究也尚未引起足够的重视. ...
Amino sugar metabolism pathway involved in chlamydospore formation of Fusarium oxysporum f. sp. cubense
1
2019a
... 尖孢镰孢菌古巴专化型的致病因子主要包括细胞壁降解酶、信号转导通路、毒素、sRNAs和致病相关基因.在病原菌和寄主互作过程中,病原菌通过分泌一系列细胞壁降解酶降解寄主植物的细胞壁,进而将多糖等营养物质从植物细胞内释放供病原菌生长,促进病原菌的侵入和定殖;多聚半乳糖醛酸酶已被鉴定为尖孢镰孢菌古巴专化型4号生理小种的致病因子(董章勇和王振中 2012).已有报道表明,FoSlt2 MAPK信号转导通路调控尖孢镰孢菌古巴专化型的生理特性和致病性(Dinget al. 2015 ;Dinget al. 2018a ;丁兆建等 2019b;Dinget al. 2020 );cAMP-PKA信号通路G蛋白亚基FGA2和FGB1参与调控尖孢镰孢菌古巴专化型的生长发育、产孢、耐热性和致病性(Guoet al. 2016 ).镰孢菌酸(fusaric acid,FA)是镰孢菌产生的非寄主专化性毒素,对动物和人类具有中低毒性,但对植物有较高的毒性,可引起多种作物的萎蔫病和根腐病害(Brownet al. 2012 ;Niehauset al. 2014 ).国内外已报道尖孢镰孢菌古巴专化型的多个致萎相关毒素,包括FA、白僵菌素和伏马菌素B1(许文耀等 2004;陈石等 2011;李春雨等 2011;Ding et al. 2018b ;Portal et al. 2018 ).关于植物病原真菌致病相关sRNAs报道较多,灰霉菌Botrytis cinerea、大丽轮枝菌Verticillium dahlia、小麦柄锈菌Puccinia striiformis f. sp. tritici和卵菌Oomycetes产生的sRNAs效应子可跨界传输到寄主细胞中,通过沉默寄主相关免疫基因,抑制寄主植物的免疫反应(Weiberget al. 2013 ;Wanget al. 2016 ;Wanget al. 2017 );关于尖孢镰孢菌古巴专化型sRNAs的研究处于起步阶段,李敏慧等(2019)发现用该病菌接种香蕉苗根部24h后,病原菌编码Dicer 和Argonaute的基因表达显著升高,sRNA测序结果也表明,大量milRNA显著高表达.也有多个相关致病基因得到鉴定,如活性氧迸发转录因子Foatf1(Qiet al. 2013 )、编码α-1,6-甘露糖转移酶的基因FoOCH1(Liet al. 2014 )、微管蛋白β1和β2(刘远征等 2018a,2018b)和Ⅴ型肌球蛋白Myosin5的协作蛋白smy1(何壮等 2019).尽管该菌的致病机理研究已取得了显著进展,但其致病机制仍不清晰,尤其对作为初侵染源的厚垣孢子的研究也尚未引起足够的重视. ...
Transcriptome analysis of the MAPK FoSlt2 deletion mutant of Fusarium oxysporum f. sp. cubense
0
2019b
A MADS-box transcription factor FoRlm1 regulates aerial hyphal growth, oxidative stress, cell wall biosynthesis and virulence in Fusarium oxysporum f. sp. cubense
1
2020
... 尖孢镰孢菌古巴专化型的致病因子主要包括细胞壁降解酶、信号转导通路、毒素、sRNAs和致病相关基因.在病原菌和寄主互作过程中,病原菌通过分泌一系列细胞壁降解酶降解寄主植物的细胞壁,进而将多糖等营养物质从植物细胞内释放供病原菌生长,促进病原菌的侵入和定殖;多聚半乳糖醛酸酶已被鉴定为尖孢镰孢菌古巴专化型4号生理小种的致病因子(董章勇和王振中 2012).已有报道表明,FoSlt2 MAPK信号转导通路调控尖孢镰孢菌古巴专化型的生理特性和致病性(Dinget al. 2015 ;Dinget al. 2018a ;丁兆建等 2019b;Dinget al. 2020 );cAMP-PKA信号通路G蛋白亚基FGA2和FGB1参与调控尖孢镰孢菌古巴专化型的生长发育、产孢、耐热性和致病性(Guoet al. 2016 ).镰孢菌酸(fusaric acid,FA)是镰孢菌产生的非寄主专化性毒素,对动物和人类具有中低毒性,但对植物有较高的毒性,可引起多种作物的萎蔫病和根腐病害(Brownet al. 2012 ;Niehauset al. 2014 ).国内外已报道尖孢镰孢菌古巴专化型的多个致萎相关毒素,包括FA、白僵菌素和伏马菌素B1(许文耀等 2004;陈石等 2011;李春雨等 2011;Ding et al. 2018b ;Portal et al. 2018 ).关于植物病原真菌致病相关sRNAs报道较多,灰霉菌Botrytis cinerea、大丽轮枝菌Verticillium dahlia、小麦柄锈菌Puccinia striiformis f. sp. tritici和卵菌Oomycetes产生的sRNAs效应子可跨界传输到寄主细胞中,通过沉默寄主相关免疫基因,抑制寄主植物的免疫反应(Weiberget al. 2013 ;Wanget al. 2016 ;Wanget al. 2017 );关于尖孢镰孢菌古巴专化型sRNAs的研究处于起步阶段,李敏慧等(2019)发现用该病菌接种香蕉苗根部24h后,病原菌编码Dicer 和Argonaute的基因表达显著升高,sRNA测序结果也表明,大量milRNA显著高表达.也有多个相关致病基因得到鉴定,如活性氧迸发转录因子Foatf1(Qiet al. 2013 )、编码α-1,6-甘露糖转移酶的基因FoOCH1(Liet al. 2014 )、微管蛋白β1和β2(刘远征等 2018a,2018b)和Ⅴ型肌球蛋白Myosin5的协作蛋白smy1(何壮等 2019).尽管该菌的致病机理研究已取得了显著进展,但其致病机制仍不清晰,尤其对作为初侵染源的厚垣孢子的研究也尚未引起足够的重视. ...
Fusaric acid is a virulence factor of Fusarium oxysporum f. sp. cubenseon banana plantlets
0
2018b
Research progress of fungal cell wall degrading enzyme
0
2012
Threonine deaminase MoIlv1 is important for conidiogenesis and pathogenesis in the rice blast fungus Magnaporthe oryzae
4
2014
... 氨基酸是重要的有机化合物,其在蛋白质合成、细胞生长、发育和能量产生等多个生物学过程中起着重要作用.已有研究证明氨基酸代谢与真菌的产孢、生长和致病性相关.在人类致病菌中,赖氨酸合成基因lysF调控烟曲霉Aspergillus fumigatus的致病性(Liebmannet al. 2004 );3个精氨酸合成相关基因Car1、Agt1和Gbu1调控白念珠菌Candida albicans的生长和致病性(Schaeferet al. 2020 );天冬氨酸合成基因asd的缺失抑制白念珠菌的生长(Dahalet al. 2020 ).L-精氨酸是生防菌盾壳霉Coniothyrium minitans产孢所必需的,其衍生物一氧化氮可能介导其产孢功能(Gonget al. 2007 ).在植物病原菌中,尖孢镰孢菌甜瓜专化型F.oxysporum f. sp. melonis精氨酸合成基因ARG1缺失突变体(Namikiet al. 2001 )和灰霉菌精氨酸合成基因bcass1缺失突变体(Patelet al. 2010 )的致病性均减弱;甲硫氨酸合成基因FgMETB调控禾谷镰孢菌的孢子萌发、菌丝生长、DON毒素产生和致病性(Fuet al. 2013 );谷氨酸合成基因P5Cdh调控栗疫病菌Cryphonectria parasitica的致病性和产孢(Yaoet al. 2013 );甲硫氨酸合成基因MET13(Yanet al. 2013 )、赖氨酸合成基因MoLys2(Chenet al. 2014 )、苏氨酸合成基因MoIlv1(Duet al. 2014 )、精氨酸合成基因(MoARG1、MoARG5、MoARG6 和 MoARG7)(Zhanget al. 2015 )、亮氨酸合成基因(MoLEU1、MoLEU2、MoLEU4、LEU1、LEU2A和LEU4)(Queet al. 2020 ;Weiet al. 2020 )与稻瘟菌的生理特性和致病性密切相关.迄今,尚未有氨基酸与尖孢镰孢菌古巴专化型产孢、生长发育和致病性的相关报道. ...
... 通过对氨基酸合成通路93个差异表达基因的In silico分析,发现其中部分差异表达基因在其他真菌已得到鉴定,如FOIG_05525、FOIG_05468、FOIG_09550和FOIG_075934个基因的同源基因参与调控真菌亮氨酸的合成(Liebmannet al. 2004 ;Queet al. 2020 );FOIG_12215和FOIG_09392的同源基因参与调控尖孢镰孢菌甜瓜专化型精氨酸的合成(Namikiet al. 2001 );FOIG_12832和FOIG_03926的同源基因参与调控白念珠菌甘氨酸的合成(McNeilet al. 2000 );FOIG_05323的同源基因参与调控烟曲霉支链氨基酸的合成(Oliveret al. 2012 );FOIG_02315的同源基因参与调控白念珠菌天冬氨酸的合成(Dahalet al. 2020 );FOIG_01066、FOIG_01154、FOIG_10878、FOIG_02315、FOIG_07232、FOIG_07170、FOIG_03322、FOIG_05025、FOIG_10638、FOIG_07565、FOIG_0314711个基因的同源基因参与调控真菌的生长发育或产孢(Puyeskyet al. 1997 ;Baidyaroyet al. 2001 ;Rodakiet al. 2006 ;Dinget al. 2010 ;Kuret al. 2010 ;Gerkeet al. 2012 ;Fuet al. 2013 ;Duet al. 2014 ;Fernandezet al. 2014 ;Dahalet al. 2020 ;Schaeferet al. 2020 );FOIG_00393、FOIG_01066、FOIG_12215、FOIG_05468、FOIG_05352、FOIG_05323、FOIG_06701、FOIG_06470、FOIG_09550、FOIG_05525、FOIG_09392、FOIG_07410、FOIG_07232、FOIG_05897、FOIG_03295、FOIG_05025、FOIG_15020、FOIG_07593、FOIG_0756519个基因的同源基因参与调控真菌的致病性或毒素产生(Chenget al. 1999 ;Baidyaroyet al. 2001 ;Namikiet al. 2001 ;Liebmannet al. 2004 ;Seonget al. 2005 ;Dinget al. 2010 ;Kingsbury & McCusker 2010;Oliveret al. 2012 ;Fuet al. 2013 ;Yaoet al. 2013 ;Duet al. 2014 ;Liet al. 2018 ;Guoet al. 2019 ;Queet al. 2020 ;Schaeferet al. 2020 ;Weiet al. 2020 )(表1).由此说明,氨基酸合成通路不仅与尖孢镰孢菌古巴专化型的氨基酸合成和厚垣孢子形成相关,其有可能参与调控该病菌的致病性. ...
... ;Duet al. 2014 ;Liet al. 2018 ;Guoet al. 2019 ;Queet al. 2020 ;Schaeferet al. 2020 ;Weiet al. 2020 )(表1).由此说明,氨基酸合成通路不仅与尖孢镰孢菌古巴专化型的氨基酸合成和厚垣孢子形成相关,其有可能参与调控该病菌的致病性. ...
... 氨基酸合成通路通过调控氨基酸的合成,影响尖孢镰孢菌古巴专化型厚垣孢子的形成.前人的研究表明,氨基酸合成基因的敲除能导致氨基酸的合成缺失,同时影响真菌生长或产孢,如白念珠菌精氨酸合成基因 Car1、Agt1和Gbu1(Schaeferet al. 2020 )及天冬氨酸合成基因asd(Dahalet al. 2020 ),禾谷镰孢菌甲硫氨酸合成基因FgMETB(Fuet al. 2013 )、栗疫病菌谷氨酸合成基因 P5Cdh(Yaoet al. 2013 )、稻瘟菌甲硫氨酸合成基因MET13(Yanet al. 2013 )、赖氨酸合成基因MoLys2(Chenet al. 2014 )、苏氨酸合成基因MoIlv1(Duet al. 2014 )、精氨酸合成基因(MoARG1、MoARG5、MoARG6和MoARG7)(Zhanget al. 2015 )、亮氨酸合成基因(MoLEU1、MoLEU2、MoLEU4、LEU1、LEU2A 和LEU4)(Queet al. 2020 ;Weiet al. 2020 ).值得指出的是,这些氨基酸合成基因的同源基因的表达水平在尖孢镰孢菌古巴专化型厚垣孢子形成过程中均发生了变化.此外,本研究发现外源甘氨酸抑制尖孢镰孢菌古巴专化型厚垣孢子的诱导形成.这些数据确认了氨基酸合成基因与尖孢镰孢菌古巴专化型厚垣孢子形成存在联系,氨基酸合成通路可通过调控氨基酸合成基因的转录,进而影响尖孢镰孢菌古巴专化型厚垣孢子的形成. ...
Evidence for a transketolase-mediated metabolic checkpoint governing biotrophic growth in rice cells by the blast fungus Magnaporthe oryzae
1
2014
... 通过对氨基酸合成通路93个差异表达基因的In silico分析,发现其中部分差异表达基因在其他真菌已得到鉴定,如FOIG_05525、FOIG_05468、FOIG_09550和FOIG_075934个基因的同源基因参与调控真菌亮氨酸的合成(Liebmannet al. 2004 ;Queet al. 2020 );FOIG_12215和FOIG_09392的同源基因参与调控尖孢镰孢菌甜瓜专化型精氨酸的合成(Namikiet al. 2001 );FOIG_12832和FOIG_03926的同源基因参与调控白念珠菌甘氨酸的合成(McNeilet al. 2000 );FOIG_05323的同源基因参与调控烟曲霉支链氨基酸的合成(Oliveret al. 2012 );FOIG_02315的同源基因参与调控白念珠菌天冬氨酸的合成(Dahalet al. 2020 );FOIG_01066、FOIG_01154、FOIG_10878、FOIG_02315、FOIG_07232、FOIG_07170、FOIG_03322、FOIG_05025、FOIG_10638、FOIG_07565、FOIG_0314711个基因的同源基因参与调控真菌的生长发育或产孢(Puyeskyet al. 1997 ;Baidyaroyet al. 2001 ;Rodakiet al. 2006 ;Dinget al. 2010 ;Kuret al. 2010 ;Gerkeet al. 2012 ;Fuet al. 2013 ;Duet al. 2014 ;Fernandezet al. 2014 ;Dahalet al. 2020 ;Schaeferet al. 2020 );FOIG_00393、FOIG_01066、FOIG_12215、FOIG_05468、FOIG_05352、FOIG_05323、FOIG_06701、FOIG_06470、FOIG_09550、FOIG_05525、FOIG_09392、FOIG_07410、FOIG_07232、FOIG_05897、FOIG_03295、FOIG_05025、FOIG_15020、FOIG_07593、FOIG_0756519个基因的同源基因参与调控真菌的致病性或毒素产生(Chenget al. 1999 ;Baidyaroyet al. 2001 ;Namikiet al. 2001 ;Liebmannet al. 2004 ;Seonget al. 2005 ;Dinget al. 2010 ;Kingsbury & McCusker 2010;Oliveret al. 2012 ;Fuet al. 2013 ;Yaoet al. 2013 ;Duet al. 2014 ;Liet al. 2018 ;Guoet al. 2019 ;Queet al. 2020 ;Schaeferet al. 2020 ;Weiet al. 2020 )(表1).由此说明,氨基酸合成通路不仅与尖孢镰孢菌古巴专化型的氨基酸合成和厚垣孢子形成相关,其有可能参与调控该病菌的致病性. ...
Cystathionine gamma-synthase is essential for methionine biosynthesis in Fusarium graminearum
4
2013
... 氨基酸是重要的有机化合物,其在蛋白质合成、细胞生长、发育和能量产生等多个生物学过程中起着重要作用.已有研究证明氨基酸代谢与真菌的产孢、生长和致病性相关.在人类致病菌中,赖氨酸合成基因lysF调控烟曲霉Aspergillus fumigatus的致病性(Liebmannet al. 2004 );3个精氨酸合成相关基因Car1、Agt1和Gbu1调控白念珠菌Candida albicans的生长和致病性(Schaeferet al. 2020 );天冬氨酸合成基因asd的缺失抑制白念珠菌的生长(Dahalet al. 2020 ).L-精氨酸是生防菌盾壳霉Coniothyrium minitans产孢所必需的,其衍生物一氧化氮可能介导其产孢功能(Gonget al. 2007 ).在植物病原菌中,尖孢镰孢菌甜瓜专化型F.oxysporum f. sp. melonis精氨酸合成基因ARG1缺失突变体(Namikiet al. 2001 )和灰霉菌精氨酸合成基因bcass1缺失突变体(Patelet al. 2010 )的致病性均减弱;甲硫氨酸合成基因FgMETB调控禾谷镰孢菌的孢子萌发、菌丝生长、DON毒素产生和致病性(Fuet al. 2013 );谷氨酸合成基因P5Cdh调控栗疫病菌Cryphonectria parasitica的致病性和产孢(Yaoet al. 2013 );甲硫氨酸合成基因MET13(Yanet al. 2013 )、赖氨酸合成基因MoLys2(Chenet al. 2014 )、苏氨酸合成基因MoIlv1(Duet al. 2014 )、精氨酸合成基因(MoARG1、MoARG5、MoARG6 和 MoARG7)(Zhanget al. 2015 )、亮氨酸合成基因(MoLEU1、MoLEU2、MoLEU4、LEU1、LEU2A和LEU4)(Queet al. 2020 ;Weiet al. 2020 )与稻瘟菌的生理特性和致病性密切相关.迄今,尚未有氨基酸与尖孢镰孢菌古巴专化型产孢、生长发育和致病性的相关报道. ...
... 通过对氨基酸合成通路93个差异表达基因的In silico分析,发现其中部分差异表达基因在其他真菌已得到鉴定,如FOIG_05525、FOIG_05468、FOIG_09550和FOIG_075934个基因的同源基因参与调控真菌亮氨酸的合成(Liebmannet al. 2004 ;Queet al. 2020 );FOIG_12215和FOIG_09392的同源基因参与调控尖孢镰孢菌甜瓜专化型精氨酸的合成(Namikiet al. 2001 );FOIG_12832和FOIG_03926的同源基因参与调控白念珠菌甘氨酸的合成(McNeilet al. 2000 );FOIG_05323的同源基因参与调控烟曲霉支链氨基酸的合成(Oliveret al. 2012 );FOIG_02315的同源基因参与调控白念珠菌天冬氨酸的合成(Dahalet al. 2020 );FOIG_01066、FOIG_01154、FOIG_10878、FOIG_02315、FOIG_07232、FOIG_07170、FOIG_03322、FOIG_05025、FOIG_10638、FOIG_07565、FOIG_0314711个基因的同源基因参与调控真菌的生长发育或产孢(Puyeskyet al. 1997 ;Baidyaroyet al. 2001 ;Rodakiet al. 2006 ;Dinget al. 2010 ;Kuret al. 2010 ;Gerkeet al. 2012 ;Fuet al. 2013 ;Duet al. 2014 ;Fernandezet al. 2014 ;Dahalet al. 2020 ;Schaeferet al. 2020 );FOIG_00393、FOIG_01066、FOIG_12215、FOIG_05468、FOIG_05352、FOIG_05323、FOIG_06701、FOIG_06470、FOIG_09550、FOIG_05525、FOIG_09392、FOIG_07410、FOIG_07232、FOIG_05897、FOIG_03295、FOIG_05025、FOIG_15020、FOIG_07593、FOIG_0756519个基因的同源基因参与调控真菌的致病性或毒素产生(Chenget al. 1999 ;Baidyaroyet al. 2001 ;Namikiet al. 2001 ;Liebmannet al. 2004 ;Seonget al. 2005 ;Dinget al. 2010 ;Kingsbury & McCusker 2010;Oliveret al. 2012 ;Fuet al. 2013 ;Yaoet al. 2013 ;Duet al. 2014 ;Liet al. 2018 ;Guoet al. 2019 ;Queet al. 2020 ;Schaeferet al. 2020 ;Weiet al. 2020 )(表1).由此说明,氨基酸合成通路不仅与尖孢镰孢菌古巴专化型的氨基酸合成和厚垣孢子形成相关,其有可能参与调控该病菌的致病性. ...
... ;Fuet al. 2013 ;Yaoet al. 2013 ;Duet al. 2014 ;Liet al. 2018 ;Guoet al. 2019 ;Queet al. 2020 ;Schaeferet al. 2020 ;Weiet al. 2020 )(表1).由此说明,氨基酸合成通路不仅与尖孢镰孢菌古巴专化型的氨基酸合成和厚垣孢子形成相关,其有可能参与调控该病菌的致病性. ...
... 氨基酸合成通路通过调控氨基酸的合成,影响尖孢镰孢菌古巴专化型厚垣孢子的形成.前人的研究表明,氨基酸合成基因的敲除能导致氨基酸的合成缺失,同时影响真菌生长或产孢,如白念珠菌精氨酸合成基因 Car1、Agt1和Gbu1(Schaeferet al. 2020 )及天冬氨酸合成基因asd(Dahalet al. 2020 ),禾谷镰孢菌甲硫氨酸合成基因FgMETB(Fuet al. 2013 )、栗疫病菌谷氨酸合成基因 P5Cdh(Yaoet al. 2013 )、稻瘟菌甲硫氨酸合成基因MET13(Yanet al. 2013 )、赖氨酸合成基因MoLys2(Chenet al. 2014 )、苏氨酸合成基因MoIlv1(Duet al. 2014 )、精氨酸合成基因(MoARG1、MoARG5、MoARG6和MoARG7)(Zhanget al. 2015 )、亮氨酸合成基因(MoLEU1、MoLEU2、MoLEU4、LEU1、LEU2A 和LEU4)(Queet al. 2020 ;Weiet al. 2020 ).值得指出的是,这些氨基酸合成基因的同源基因的表达水平在尖孢镰孢菌古巴专化型厚垣孢子形成过程中均发生了变化.此外,本研究发现外源甘氨酸抑制尖孢镰孢菌古巴专化型厚垣孢子的诱导形成.这些数据确认了氨基酸合成基因与尖孢镰孢菌古巴专化型厚垣孢子形成存在联系,氨基酸合成通路可通过调控氨基酸合成基因的转录,进而影响尖孢镰孢菌古巴专化型厚垣孢子的形成. ...
Fungal S-adenosylmethionine synthetase and the control of development and secondary metabolism in Aspergillus nidulans
1
2012
... 通过对氨基酸合成通路93个差异表达基因的In silico分析,发现其中部分差异表达基因在其他真菌已得到鉴定,如FOIG_05525、FOIG_05468、FOIG_09550和FOIG_075934个基因的同源基因参与调控真菌亮氨酸的合成(Liebmannet al. 2004 ;Queet al. 2020 );FOIG_12215和FOIG_09392的同源基因参与调控尖孢镰孢菌甜瓜专化型精氨酸的合成(Namikiet al. 2001 );FOIG_12832和FOIG_03926的同源基因参与调控白念珠菌甘氨酸的合成(McNeilet al. 2000 );FOIG_05323的同源基因参与调控烟曲霉支链氨基酸的合成(Oliveret al. 2012 );FOIG_02315的同源基因参与调控白念珠菌天冬氨酸的合成(Dahalet al. 2020 );FOIG_01066、FOIG_01154、FOIG_10878、FOIG_02315、FOIG_07232、FOIG_07170、FOIG_03322、FOIG_05025、FOIG_10638、FOIG_07565、FOIG_0314711个基因的同源基因参与调控真菌的生长发育或产孢(Puyeskyet al. 1997 ;Baidyaroyet al. 2001 ;Rodakiet al. 2006 ;Dinget al. 2010 ;Kuret al. 2010 ;Gerkeet al. 2012 ;Fuet al. 2013 ;Duet al. 2014 ;Fernandezet al. 2014 ;Dahalet al. 2020 ;Schaeferet al. 2020 );FOIG_00393、FOIG_01066、FOIG_12215、FOIG_05468、FOIG_05352、FOIG_05323、FOIG_06701、FOIG_06470、FOIG_09550、FOIG_05525、FOIG_09392、FOIG_07410、FOIG_07232、FOIG_05897、FOIG_03295、FOIG_05025、FOIG_15020、FOIG_07593、FOIG_0756519个基因的同源基因参与调控真菌的致病性或毒素产生(Chenget al. 1999 ;Baidyaroyet al. 2001 ;Namikiet al. 2001 ;Liebmannet al. 2004 ;Seonget al. 2005 ;Dinget al. 2010 ;Kingsbury & McCusker 2010;Oliveret al. 2012 ;Fuet al. 2013 ;Yaoet al. 2013 ;Duet al. 2014 ;Liet al. 2018 ;Guoet al. 2019 ;Queet al. 2020 ;Schaeferet al. 2020 ;Weiet al. 2020 )(表1).由此说明,氨基酸合成通路不仅与尖孢镰孢菌古巴专化型的氨基酸合成和厚垣孢子形成相关,其有可能参与调控该病菌的致病性. ...
L-arginine is essential for conidiation in the filamentous fungus Coniothyrium minitans
1
2007
... 氨基酸是重要的有机化合物,其在蛋白质合成、细胞生长、发育和能量产生等多个生物学过程中起着重要作用.已有研究证明氨基酸代谢与真菌的产孢、生长和致病性相关.在人类致病菌中,赖氨酸合成基因lysF调控烟曲霉Aspergillus fumigatus的致病性(Liebmannet al. 2004 );3个精氨酸合成相关基因Car1、Agt1和Gbu1调控白念珠菌Candida albicans的生长和致病性(Schaeferet al. 2020 );天冬氨酸合成基因asd的缺失抑制白念珠菌的生长(Dahalet al. 2020 ).L-精氨酸是生防菌盾壳霉Coniothyrium minitans产孢所必需的,其衍生物一氧化氮可能介导其产孢功能(Gonget al. 2007 ).在植物病原菌中,尖孢镰孢菌甜瓜专化型F.oxysporum f. sp. melonis精氨酸合成基因ARG1缺失突变体(Namikiet al. 2001 )和灰霉菌精氨酸合成基因bcass1缺失突变体(Patelet al. 2010 )的致病性均减弱;甲硫氨酸合成基因FgMETB调控禾谷镰孢菌的孢子萌发、菌丝生长、DON毒素产生和致病性(Fuet al. 2013 );谷氨酸合成基因P5Cdh调控栗疫病菌Cryphonectria parasitica的致病性和产孢(Yaoet al. 2013 );甲硫氨酸合成基因MET13(Yanet al. 2013 )、赖氨酸合成基因MoLys2(Chenet al. 2014 )、苏氨酸合成基因MoIlv1(Duet al. 2014 )、精氨酸合成基因(MoARG1、MoARG5、MoARG6 和 MoARG7)(Zhanget al. 2015 )、亮氨酸合成基因(MoLEU1、MoLEU2、MoLEU4、LEU1、LEU2A和LEU4)(Queet al. 2020 ;Weiet al. 2020 )与稻瘟菌的生理特性和致病性密切相关.迄今,尚未有氨基酸与尖孢镰孢菌古巴专化型产孢、生长发育和致病性的相关报道. ...
Roles of low pH, carbon and inorganic nitrogen source use in chlamydospore formation byFusarium solani
1
1976
... 氨基酸如何影响真菌产孢、生长或者抑制尖孢镰孢菌古巴专化型厚垣孢子形成呢?真菌为适应不同或者变化的环境,会尽可能广泛地选择营养来源.有些真菌种类利用氨基酸的降解产物作为唯一氮源,有的则利用其作为唯一碳源,有的则两者兼有之(Rzadet al. 2018 ),如白念珠菌、新型隐球菌Cryptococcus neoformans和烟曲霉利用赖氨酸作为唯一氮源,酿酒酵母则利用赖氨酸作为唯一的碳源而非唯一氮源(Kinzelet al. 1983 ).已有报道表明,碳源的浓度与真菌厚垣孢子的形成相关,高浓度的葡萄糖抑制白色念珠菌和镰孢菌厚垣孢子的形成,缺乏或者低浓度的葡萄糖可促使镰孢菌厚垣孢子的形成(Qureshi & Page 1970;Griffin 1976;Mandal & Chaudhuri 2013;Bottcheret al. 2016 ).前期的研究发现,作为碳源的N-乙酰葡糖胺浓度达到20g/L时,尖孢镰孢菌古巴专化型的菌丝不产生厚垣孢子;当N-乙酰葡糖胺缺乏时,其菌丝可产生大量厚垣孢子(丁兆建等 2019a).由此推测,外源甘氨酸的添加导致碳源的浓度升高,从而抑制尖孢镰孢菌古巴专化型厚垣孢子的诱导形成. ...
The G-protein subunits FGA2 and FGB1 play distinct roles in development and pathogenicity in the banana fungal pathogen Fusarium oxysporum f. sp. cubense
1
2016
... 尖孢镰孢菌古巴专化型的致病因子主要包括细胞壁降解酶、信号转导通路、毒素、sRNAs和致病相关基因.在病原菌和寄主互作过程中,病原菌通过分泌一系列细胞壁降解酶降解寄主植物的细胞壁,进而将多糖等营养物质从植物细胞内释放供病原菌生长,促进病原菌的侵入和定殖;多聚半乳糖醛酸酶已被鉴定为尖孢镰孢菌古巴专化型4号生理小种的致病因子(董章勇和王振中 2012).已有报道表明,FoSlt2 MAPK信号转导通路调控尖孢镰孢菌古巴专化型的生理特性和致病性(Dinget al. 2015 ;Dinget al. 2018a ;丁兆建等 2019b;Dinget al. 2020 );cAMP-PKA信号通路G蛋白亚基FGA2和FGB1参与调控尖孢镰孢菌古巴专化型的生长发育、产孢、耐热性和致病性(Guoet al. 2016 ).镰孢菌酸(fusaric acid,FA)是镰孢菌产生的非寄主专化性毒素,对动物和人类具有中低毒性,但对植物有较高的毒性,可引起多种作物的萎蔫病和根腐病害(Brownet al. 2012 ;Niehauset al. 2014 ).国内外已报道尖孢镰孢菌古巴专化型的多个致萎相关毒素,包括FA、白僵菌素和伏马菌素B1(许文耀等 2004;陈石等 2011;李春雨等 2011;Ding et al. 2018b ;Portal et al. 2018 ).关于植物病原真菌致病相关sRNAs报道较多,灰霉菌Botrytis cinerea、大丽轮枝菌Verticillium dahlia、小麦柄锈菌Puccinia striiformis f. sp. tritici和卵菌Oomycetes产生的sRNAs效应子可跨界传输到寄主细胞中,通过沉默寄主相关免疫基因,抑制寄主植物的免疫反应(Weiberget al. 2013 ;Wanget al. 2016 ;Wanget al. 2017 );关于尖孢镰孢菌古巴专化型sRNAs的研究处于起步阶段,李敏慧等(2019)发现用该病菌接种香蕉苗根部24h后,病原菌编码Dicer 和Argonaute的基因表达显著升高,sRNA测序结果也表明,大量milRNA显著高表达.也有多个相关致病基因得到鉴定,如活性氧迸发转录因子Foatf1(Qiet al. 2013 )、编码α-1,6-甘露糖转移酶的基因FoOCH1(Liet al. 2014 )、微管蛋白β1和β2(刘远征等 2018a,2018b)和Ⅴ型肌球蛋白Myosin5的协作蛋白smy1(何壮等 2019).尽管该菌的致病机理研究已取得了显著进展,但其致病机制仍不清晰,尤其对作为初侵染源的厚垣孢子的研究也尚未引起足够的重视. ...
Functional characterization of a cystathionine beta-synthase gene in sulfur metabolism and pathogenicity of Aspergillus niger in pear fruit
1
2019
... 通过对氨基酸合成通路93个差异表达基因的In silico分析,发现其中部分差异表达基因在其他真菌已得到鉴定,如FOIG_05525、FOIG_05468、FOIG_09550和FOIG_075934个基因的同源基因参与调控真菌亮氨酸的合成(Liebmannet al. 2004 ;Queet al. 2020 );FOIG_12215和FOIG_09392的同源基因参与调控尖孢镰孢菌甜瓜专化型精氨酸的合成(Namikiet al. 2001 );FOIG_12832和FOIG_03926的同源基因参与调控白念珠菌甘氨酸的合成(McNeilet al. 2000 );FOIG_05323的同源基因参与调控烟曲霉支链氨基酸的合成(Oliveret al. 2012 );FOIG_02315的同源基因参与调控白念珠菌天冬氨酸的合成(Dahalet al. 2020 );FOIG_01066、FOIG_01154、FOIG_10878、FOIG_02315、FOIG_07232、FOIG_07170、FOIG_03322、FOIG_05025、FOIG_10638、FOIG_07565、FOIG_0314711个基因的同源基因参与调控真菌的生长发育或产孢(Puyeskyet al. 1997 ;Baidyaroyet al. 2001 ;Rodakiet al. 2006 ;Dinget al. 2010 ;Kuret al. 2010 ;Gerkeet al. 2012 ;Fuet al. 2013 ;Duet al. 2014 ;Fernandezet al. 2014 ;Dahalet al. 2020 ;Schaeferet al. 2020 );FOIG_00393、FOIG_01066、FOIG_12215、FOIG_05468、FOIG_05352、FOIG_05323、FOIG_06701、FOIG_06470、FOIG_09550、FOIG_05525、FOIG_09392、FOIG_07410、FOIG_07232、FOIG_05897、FOIG_03295、FOIG_05025、FOIG_15020、FOIG_07593、FOIG_0756519个基因的同源基因参与调控真菌的致病性或毒素产生(Chenget al. 1999 ;Baidyaroyet al. 2001 ;Namikiet al. 2001 ;Liebmannet al. 2004 ;Seonget al. 2005 ;Dinget al. 2010 ;Kingsbury & McCusker 2010;Oliveret al. 2012 ;Fuet al. 2013 ;Yaoet al. 2013 ;Duet al. 2014 ;Liet al. 2018 ;Guoet al. 2019 ;Queet al. 2020 ;Schaeferet al. 2020 ;Weiet al. 2020 )(表1).由此说明,氨基酸合成通路不仅与尖孢镰孢菌古巴专化型的氨基酸合成和厚垣孢子形成相关,其有可能参与调控该病菌的致病性. ...
Effects of smy1 gene knockout on physiological function of Fusarium oxysporum f. sp. cubense race 4
0
2019
Sporulation of Fusarium oxysporum f. sp. lycopersici on stem surfaces of tomato plants and aerial dissemination of inoculum
1
1997
... 香蕉枯萎病是由尖孢镰孢菌古巴专化型 Fusarium oxysporum f. sp. cubense侵染引起的一种土传真菌病害,现已成为热带和亚热带地区香蕉产业的重大威胁(Ploetz 2006).根据对不同香蕉品种的致病力差异分析,该病菌被分为3个生理小种(1、2和4号生理小种),其中1号生理小种侵染大密哈(Gros Michel,AAA)和基因型为AAB的香蕉品种;2号生理小种侵染棱香蕉(Bluggoe,AAB);4号生理小种几乎侵染所有的香蕉品种,给种植户造成了严重的经济损失(Su et al. 1986).该病菌产生3种类型的孢子,分别为大型分生孢子、小型分生孢子和厚垣孢子;其中大型分生孢子和小型分生孢子是次级侵染源,多形成于香蕉寄主根部和假茎的表面;厚垣孢子是一种厚壁休眠孢子,是初级侵染源,其可在土壤中存活多年甚至10年以上.当厚垣孢子受到香蕉根部分泌物的信号刺激后,会萌发形成菌丝,侵染香蕉寄主,导致发生香蕉枯萎病(Katan et al. 1997;Ohara & Tsuge 2004;丁兆建等2019a). ...
Fungal homoserine kinase (thr1Δ) mutants are attenuated in virulence and die rapidly upon threonine starvation and serum incubation
1
2010
... 通过对氨基酸合成通路93个差异表达基因的In silico分析,发现其中部分差异表达基因在其他真菌已得到鉴定,如FOIG_05525、FOIG_05468、FOIG_09550和FOIG_075934个基因的同源基因参与调控真菌亮氨酸的合成(Liebmannet al. 2004 ;Queet al. 2020 );FOIG_12215和FOIG_09392的同源基因参与调控尖孢镰孢菌甜瓜专化型精氨酸的合成(Namikiet al. 2001 );FOIG_12832和FOIG_03926的同源基因参与调控白念珠菌甘氨酸的合成(McNeilet al. 2000 );FOIG_05323的同源基因参与调控烟曲霉支链氨基酸的合成(Oliveret al. 2012 );FOIG_02315的同源基因参与调控白念珠菌天冬氨酸的合成(Dahalet al. 2020 );FOIG_01066、FOIG_01154、FOIG_10878、FOIG_02315、FOIG_07232、FOIG_07170、FOIG_03322、FOIG_05025、FOIG_10638、FOIG_07565、FOIG_0314711个基因的同源基因参与调控真菌的生长发育或产孢(Puyeskyet al. 1997 ;Baidyaroyet al. 2001 ;Rodakiet al. 2006 ;Dinget al. 2010 ;Kuret al. 2010 ;Gerkeet al. 2012 ;Fuet al. 2013 ;Duet al. 2014 ;Fernandezet al. 2014 ;Dahalet al. 2020 ;Schaeferet al. 2020 );FOIG_00393、FOIG_01066、FOIG_12215、FOIG_05468、FOIG_05352、FOIG_05323、FOIG_06701、FOIG_06470、FOIG_09550、FOIG_05525、FOIG_09392、FOIG_07410、FOIG_07232、FOIG_05897、FOIG_03295、FOIG_05025、FOIG_15020、FOIG_07593、FOIG_0756519个基因的同源基因参与调控真菌的致病性或毒素产生(Chenget al. 1999 ;Baidyaroyet al. 2001 ;Namikiet al. 2001 ;Liebmannet al. 2004 ;Seonget al. 2005 ;Dinget al. 2010 ;Kingsbury & McCusker 2010;Oliveret al. 2012 ;Fuet al. 2013 ;Yaoet al. 2013 ;Duet al. 2014 ;Liet al. 2018 ;Guoet al. 2019 ;Queet al. 2020 ;Schaeferet al. 2020 ;Weiet al. 2020 )(表1).由此说明,氨基酸合成通路不仅与尖孢镰孢菌古巴专化型的氨基酸合成和厚垣孢子形成相关,其有可能参与调控该病菌的致病性. ...
Role of L-lysine-alpha-ketoglutarate aminotransferase in catabolism of lysine as a nitrogen source forRhodotorula glutinis
1
1983
... 氨基酸如何影响真菌产孢、生长或者抑制尖孢镰孢菌古巴专化型厚垣孢子形成呢?真菌为适应不同或者变化的环境,会尽可能广泛地选择营养来源.有些真菌种类利用氨基酸的降解产物作为唯一氮源,有的则利用其作为唯一碳源,有的则两者兼有之(Rzadet al. 2018 ),如白念珠菌、新型隐球菌Cryptococcus neoformans和烟曲霉利用赖氨酸作为唯一氮源,酿酒酵母则利用赖氨酸作为唯一的碳源而非唯一氮源(Kinzelet al. 1983 ).已有报道表明,碳源的浓度与真菌厚垣孢子的形成相关,高浓度的葡萄糖抑制白色念珠菌和镰孢菌厚垣孢子的形成,缺乏或者低浓度的葡萄糖可促使镰孢菌厚垣孢子的形成(Qureshi & Page 1970;Griffin 1976;Mandal & Chaudhuri 2013;Bottcheret al. 2016 ).前期的研究发现,作为碳源的N-乙酰葡糖胺浓度达到20g/L时,尖孢镰孢菌古巴专化型的菌丝不产生厚垣孢子;当N-乙酰葡糖胺缺乏时,其菌丝可产生大量厚垣孢子(丁兆建等 2019a).由此推测,外源甘氨酸的添加导致碳源的浓度升高,从而抑制尖孢镰孢菌古巴专化型厚垣孢子的诱导形成. ...
Disruption of homocitrate synthase genes in Candida albicans affects growth but not virulence
1
2010
... 通过对氨基酸合成通路93个差异表达基因的In silico分析,发现其中部分差异表达基因在其他真菌已得到鉴定,如FOIG_05525、FOIG_05468、FOIG_09550和FOIG_075934个基因的同源基因参与调控真菌亮氨酸的合成(Liebmannet al. 2004 ;Queet al. 2020 );FOIG_12215和FOIG_09392的同源基因参与调控尖孢镰孢菌甜瓜专化型精氨酸的合成(Namikiet al. 2001 );FOIG_12832和FOIG_03926的同源基因参与调控白念珠菌甘氨酸的合成(McNeilet al. 2000 );FOIG_05323的同源基因参与调控烟曲霉支链氨基酸的合成(Oliveret al. 2012 );FOIG_02315的同源基因参与调控白念珠菌天冬氨酸的合成(Dahalet al. 2020 );FOIG_01066、FOIG_01154、FOIG_10878、FOIG_02315、FOIG_07232、FOIG_07170、FOIG_03322、FOIG_05025、FOIG_10638、FOIG_07565、FOIG_0314711个基因的同源基因参与调控真菌的生长发育或产孢(Puyeskyet al. 1997 ;Baidyaroyet al. 2001 ;Rodakiet al. 2006 ;Dinget al. 2010 ;Kuret al. 2010 ;Gerkeet al. 2012 ;Fuet al. 2013 ;Duet al. 2014 ;Fernandezet al. 2014 ;Dahalet al. 2020 ;Schaeferet al. 2020 );FOIG_00393、FOIG_01066、FOIG_12215、FOIG_05468、FOIG_05352、FOIG_05323、FOIG_06701、FOIG_06470、FOIG_09550、FOIG_05525、FOIG_09392、FOIG_07410、FOIG_07232、FOIG_05897、FOIG_03295、FOIG_05025、FOIG_15020、FOIG_07593、FOIG_0756519个基因的同源基因参与调控真菌的致病性或毒素产生(Chenget al. 1999 ;Baidyaroyet al. 2001 ;Namikiet al. 2001 ;Liebmannet al. 2004 ;Seonget al. 2005 ;Dinget al. 2010 ;Kingsbury & McCusker 2010;Oliveret al. 2012 ;Fuet al. 2013 ;Yaoet al. 2013 ;Duet al. 2014 ;Liet al. 2018 ;Guoet al. 2019 ;Queet al. 2020 ;Schaeferet al. 2020 ;Weiet al. 2020 )(表1).由此说明,氨基酸合成通路不仅与尖孢镰孢菌古巴专化型的氨基酸合成和厚垣孢子形成相关,其有可能参与调控该病菌的致病性. ...
Identification of beauvericin, a novel mycotoxin from Fusarium oxysporum f. sp. cubense
0
2011
A novel citrate synthase isoform contributes infection and stress resistance of the stripe rust fungus
1
2018
... 通过对氨基酸合成通路93个差异表达基因的In silico分析,发现其中部分差异表达基因在其他真菌已得到鉴定,如FOIG_05525、FOIG_05468、FOIG_09550和FOIG_075934个基因的同源基因参与调控真菌亮氨酸的合成(Liebmannet al. 2004 ;Queet al. 2020 );FOIG_12215和FOIG_09392的同源基因参与调控尖孢镰孢菌甜瓜专化型精氨酸的合成(Namikiet al. 2001 );FOIG_12832和FOIG_03926的同源基因参与调控白念珠菌甘氨酸的合成(McNeilet al. 2000 );FOIG_05323的同源基因参与调控烟曲霉支链氨基酸的合成(Oliveret al. 2012 );FOIG_02315的同源基因参与调控白念珠菌天冬氨酸的合成(Dahalet al. 2020 );FOIG_01066、FOIG_01154、FOIG_10878、FOIG_02315、FOIG_07232、FOIG_07170、FOIG_03322、FOIG_05025、FOIG_10638、FOIG_07565、FOIG_0314711个基因的同源基因参与调控真菌的生长发育或产孢(Puyeskyet al. 1997 ;Baidyaroyet al. 2001 ;Rodakiet al. 2006 ;Dinget al. 2010 ;Kuret al. 2010 ;Gerkeet al. 2012 ;Fuet al. 2013 ;Duet al. 2014 ;Fernandezet al. 2014 ;Dahalet al. 2020 ;Schaeferet al. 2020 );FOIG_00393、FOIG_01066、FOIG_12215、FOIG_05468、FOIG_05352、FOIG_05323、FOIG_06701、FOIG_06470、FOIG_09550、FOIG_05525、FOIG_09392、FOIG_07410、FOIG_07232、FOIG_05897、FOIG_03295、FOIG_05025、FOIG_15020、FOIG_07593、FOIG_0756519个基因的同源基因参与调控真菌的致病性或毒素产生(Chenget al. 1999 ;Baidyaroyet al. 2001 ;Namikiet al. 2001 ;Liebmannet al. 2004 ;Seonget al. 2005 ;Dinget al. 2010 ;Kingsbury & McCusker 2010;Oliveret al. 2012 ;Fuet al. 2013 ;Yaoet al. 2013 ;Duet al. 2014 ;Liet al. 2018 ;Guoet al. 2019 ;Queet al. 2020 ;Schaeferet al. 2020 ;Weiet al. 2020 )(表1).由此说明,氨基酸合成通路不仅与尖孢镰孢菌古巴专化型的氨基酸合成和厚垣孢子形成相关,其有可能参与调控该病菌的致病性. ...
Research progress in pathogenic mechanism ofFusarium oxysporum f. sp. cubense
0
2019
Functional characterization of the gene FoOCH1 encoding a putative alpha-1,6-mannosyltransferase in Fusarium oxysporum f. sp. cubense
1
2014
... 尖孢镰孢菌古巴专化型的致病因子主要包括细胞壁降解酶、信号转导通路、毒素、sRNAs和致病相关基因.在病原菌和寄主互作过程中,病原菌通过分泌一系列细胞壁降解酶降解寄主植物的细胞壁,进而将多糖等营养物质从植物细胞内释放供病原菌生长,促进病原菌的侵入和定殖;多聚半乳糖醛酸酶已被鉴定为尖孢镰孢菌古巴专化型4号生理小种的致病因子(董章勇和王振中 2012).已有报道表明,FoSlt2 MAPK信号转导通路调控尖孢镰孢菌古巴专化型的生理特性和致病性(Dinget al. 2015 ;Dinget al. 2018a ;丁兆建等 2019b;Dinget al. 2020 );cAMP-PKA信号通路G蛋白亚基FGA2和FGB1参与调控尖孢镰孢菌古巴专化型的生长发育、产孢、耐热性和致病性(Guoet al. 2016 ).镰孢菌酸(fusaric acid,FA)是镰孢菌产生的非寄主专化性毒素,对动物和人类具有中低毒性,但对植物有较高的毒性,可引起多种作物的萎蔫病和根腐病害(Brownet al. 2012 ;Niehauset al. 2014 ).国内外已报道尖孢镰孢菌古巴专化型的多个致萎相关毒素,包括FA、白僵菌素和伏马菌素B1(许文耀等 2004;陈石等 2011;李春雨等 2011;Ding et al. 2018b ;Portal et al. 2018 ).关于植物病原真菌致病相关sRNAs报道较多,灰霉菌Botrytis cinerea、大丽轮枝菌Verticillium dahlia、小麦柄锈菌Puccinia striiformis f. sp. tritici和卵菌Oomycetes产生的sRNAs效应子可跨界传输到寄主细胞中,通过沉默寄主相关免疫基因,抑制寄主植物的免疫反应(Weiberget al. 2013 ;Wanget al. 2016 ;Wanget al. 2017 );关于尖孢镰孢菌古巴专化型sRNAs的研究处于起步阶段,李敏慧等(2019)发现用该病菌接种香蕉苗根部24h后,病原菌编码Dicer 和Argonaute的基因表达显著升高,sRNA测序结果也表明,大量milRNA显著高表达.也有多个相关致病基因得到鉴定,如活性氧迸发转录因子Foatf1(Qiet al. 2013 )、编码α-1,6-甘露糖转移酶的基因FoOCH1(Liet al. 2014 )、微管蛋白β1和β2(刘远征等 2018a,2018b)和Ⅴ型肌球蛋白Myosin5的协作蛋白smy1(何壮等 2019).尽管该菌的致病机理研究已取得了显著进展,但其致病机制仍不清晰,尤其对作为初侵染源的厚垣孢子的研究也尚未引起足够的重视. ...
Deletion of the Aspergillus fumigatus lysine biosynthesis gene lysF encoding homoaconitase leads to attenuated virulence in a low-dose mouse infection model of invasive aspergillosis
3
2004
... 氨基酸是重要的有机化合物,其在蛋白质合成、细胞生长、发育和能量产生等多个生物学过程中起着重要作用.已有研究证明氨基酸代谢与真菌的产孢、生长和致病性相关.在人类致病菌中,赖氨酸合成基因lysF调控烟曲霉Aspergillus fumigatus的致病性(Liebmannet al. 2004 );3个精氨酸合成相关基因Car1、Agt1和Gbu1调控白念珠菌Candida albicans的生长和致病性(Schaeferet al. 2020 );天冬氨酸合成基因asd的缺失抑制白念珠菌的生长(Dahalet al. 2020 ).L-精氨酸是生防菌盾壳霉Coniothyrium minitans产孢所必需的,其衍生物一氧化氮可能介导其产孢功能(Gonget al. 2007 ).在植物病原菌中,尖孢镰孢菌甜瓜专化型F.oxysporum f. sp. melonis精氨酸合成基因ARG1缺失突变体(Namikiet al. 2001 )和灰霉菌精氨酸合成基因bcass1缺失突变体(Patelet al. 2010 )的致病性均减弱;甲硫氨酸合成基因FgMETB调控禾谷镰孢菌的孢子萌发、菌丝生长、DON毒素产生和致病性(Fuet al. 2013 );谷氨酸合成基因P5Cdh调控栗疫病菌Cryphonectria parasitica的致病性和产孢(Yaoet al. 2013 );甲硫氨酸合成基因MET13(Yanet al. 2013 )、赖氨酸合成基因MoLys2(Chenet al. 2014 )、苏氨酸合成基因MoIlv1(Duet al. 2014 )、精氨酸合成基因(MoARG1、MoARG5、MoARG6 和 MoARG7)(Zhanget al. 2015 )、亮氨酸合成基因(MoLEU1、MoLEU2、MoLEU4、LEU1、LEU2A和LEU4)(Queet al. 2020 ;Weiet al. 2020 )与稻瘟菌的生理特性和致病性密切相关.迄今,尚未有氨基酸与尖孢镰孢菌古巴专化型产孢、生长发育和致病性的相关报道. ...
... 通过对氨基酸合成通路93个差异表达基因的In silico分析,发现其中部分差异表达基因在其他真菌已得到鉴定,如FOIG_05525、FOIG_05468、FOIG_09550和FOIG_075934个基因的同源基因参与调控真菌亮氨酸的合成(Liebmannet al. 2004 ;Queet al. 2020 );FOIG_12215和FOIG_09392的同源基因参与调控尖孢镰孢菌甜瓜专化型精氨酸的合成(Namikiet al. 2001 );FOIG_12832和FOIG_03926的同源基因参与调控白念珠菌甘氨酸的合成(McNeilet al. 2000 );FOIG_05323的同源基因参与调控烟曲霉支链氨基酸的合成(Oliveret al. 2012 );FOIG_02315的同源基因参与调控白念珠菌天冬氨酸的合成(Dahalet al. 2020 );FOIG_01066、FOIG_01154、FOIG_10878、FOIG_02315、FOIG_07232、FOIG_07170、FOIG_03322、FOIG_05025、FOIG_10638、FOIG_07565、FOIG_0314711个基因的同源基因参与调控真菌的生长发育或产孢(Puyeskyet al. 1997 ;Baidyaroyet al. 2001 ;Rodakiet al. 2006 ;Dinget al. 2010 ;Kuret al. 2010 ;Gerkeet al. 2012 ;Fuet al. 2013 ;Duet al. 2014 ;Fernandezet al. 2014 ;Dahalet al. 2020 ;Schaeferet al. 2020 );FOIG_00393、FOIG_01066、FOIG_12215、FOIG_05468、FOIG_05352、FOIG_05323、FOIG_06701、FOIG_06470、FOIG_09550、FOIG_05525、FOIG_09392、FOIG_07410、FOIG_07232、FOIG_05897、FOIG_03295、FOIG_05025、FOIG_15020、FOIG_07593、FOIG_0756519个基因的同源基因参与调控真菌的致病性或毒素产生(Chenget al. 1999 ;Baidyaroyet al. 2001 ;Namikiet al. 2001 ;Liebmannet al. 2004 ;Seonget al. 2005 ;Dinget al. 2010 ;Kingsbury & McCusker 2010;Oliveret al. 2012 ;Fuet al. 2013 ;Yaoet al. 2013 ;Duet al. 2014 ;Liet al. 2018 ;Guoet al. 2019 ;Queet al. 2020 ;Schaeferet al. 2020 ;Weiet al. 2020 )(表1).由此说明,氨基酸合成通路不仅与尖孢镰孢菌古巴专化型的氨基酸合成和厚垣孢子形成相关,其有可能参与调控该病菌的致病性. ...
... ;Liebmannet al. 2004 ;Seonget al. 2005 ;Dinget al. 2010 ;Kingsbury & McCusker 2010;Oliveret al. 2012 ;Fuet al. 2013 ;Yaoet al. 2013 ;Duet al. 2014 ;Liet al. 2018 ;Guoet al. 2019 ;Queet al. 2020 ;Schaeferet al. 2020 ;Weiet al. 2020 )(表1).由此说明,氨基酸合成通路不仅与尖孢镰孢菌古巴专化型的氨基酸合成和厚垣孢子形成相关,其有可能参与调控该病菌的致病性. ...
Functional analysis of the β1-tubulin gene in Fusarium oxysporum f. sp. cubenserace 4
0
2018a
Gene knockout and phenotype analysis of the β2-tubulin in Fusarium oxysporum f. sp. cubense race 4
0
2018b
Induction of chlamydospore in Fusarium moniliforme
1
2013
... 氨基酸如何影响真菌产孢、生长或者抑制尖孢镰孢菌古巴专化型厚垣孢子形成呢?真菌为适应不同或者变化的环境,会尽可能广泛地选择营养来源.有些真菌种类利用氨基酸的降解产物作为唯一氮源,有的则利用其作为唯一碳源,有的则两者兼有之(Rzadet al. 2018 ),如白念珠菌、新型隐球菌Cryptococcus neoformans和烟曲霉利用赖氨酸作为唯一氮源,酿酒酵母则利用赖氨酸作为唯一的碳源而非唯一氮源(Kinzelet al. 1983 ).已有报道表明,碳源的浓度与真菌厚垣孢子的形成相关,高浓度的葡萄糖抑制白色念珠菌和镰孢菌厚垣孢子的形成,缺乏或者低浓度的葡萄糖可促使镰孢菌厚垣孢子的形成(Qureshi & Page 1970;Griffin 1976;Mandal & Chaudhuri 2013;Bottcheret al. 2016 ).前期的研究发现,作为碳源的N-乙酰葡糖胺浓度达到20g/L时,尖孢镰孢菌古巴专化型的菌丝不产生厚垣孢子;当N-乙酰葡糖胺缺乏时,其菌丝可产生大量厚垣孢子(丁兆建等 2019a).由此推测,外源甘氨酸的添加导致碳源的浓度升高,从而抑制尖孢镰孢菌古巴专化型厚垣孢子的诱导形成. ...
Glycine metabolism in Candida albicans: characterization of the serine hydroxymethyltransferase (SHM1, SHM2) and threonine aldolase (GLY1) genes
1
2000
... 通过对氨基酸合成通路93个差异表达基因的In silico分析,发现其中部分差异表达基因在其他真菌已得到鉴定,如FOIG_05525、FOIG_05468、FOIG_09550和FOIG_075934个基因的同源基因参与调控真菌亮氨酸的合成(Liebmannet al. 2004 ;Queet al. 2020 );FOIG_12215和FOIG_09392的同源基因参与调控尖孢镰孢菌甜瓜专化型精氨酸的合成(Namikiet al. 2001 );FOIG_12832和FOIG_03926的同源基因参与调控白念珠菌甘氨酸的合成(McNeilet al. 2000 );FOIG_05323的同源基因参与调控烟曲霉支链氨基酸的合成(Oliveret al. 2012 );FOIG_02315的同源基因参与调控白念珠菌天冬氨酸的合成(Dahalet al. 2020 );FOIG_01066、FOIG_01154、FOIG_10878、FOIG_02315、FOIG_07232、FOIG_07170、FOIG_03322、FOIG_05025、FOIG_10638、FOIG_07565、FOIG_0314711个基因的同源基因参与调控真菌的生长发育或产孢(Puyeskyet al. 1997 ;Baidyaroyet al. 2001 ;Rodakiet al. 2006 ;Dinget al. 2010 ;Kuret al. 2010 ;Gerkeet al. 2012 ;Fuet al. 2013 ;Duet al. 2014 ;Fernandezet al. 2014 ;Dahalet al. 2020 ;Schaeferet al. 2020 );FOIG_00393、FOIG_01066、FOIG_12215、FOIG_05468、FOIG_05352、FOIG_05323、FOIG_06701、FOIG_06470、FOIG_09550、FOIG_05525、FOIG_09392、FOIG_07410、FOIG_07232、FOIG_05897、FOIG_03295、FOIG_05025、FOIG_15020、FOIG_07593、FOIG_0756519个基因的同源基因参与调控真菌的致病性或毒素产生(Chenget al. 1999 ;Baidyaroyet al. 2001 ;Namikiet al. 2001 ;Liebmannet al. 2004 ;Seonget al. 2005 ;Dinget al. 2010 ;Kingsbury & McCusker 2010;Oliveret al. 2012 ;Fuet al. 2013 ;Yaoet al. 2013 ;Duet al. 2014 ;Liet al. 2018 ;Guoet al. 2019 ;Queet al. 2020 ;Schaeferet al. 2020 ;Weiet al. 2020 )(表1).由此说明,氨基酸合成通路不仅与尖孢镰孢菌古巴专化型的氨基酸合成和厚垣孢子形成相关,其有可能参与调控该病菌的致病性. ...
Mutation of an arginine biosynthesis gene causes reduced pathogenicity in Fusarium oxysporum f. sp. melonis
3
2001
... 氨基酸是重要的有机化合物,其在蛋白质合成、细胞生长、发育和能量产生等多个生物学过程中起着重要作用.已有研究证明氨基酸代谢与真菌的产孢、生长和致病性相关.在人类致病菌中,赖氨酸合成基因lysF调控烟曲霉Aspergillus fumigatus的致病性(Liebmannet al. 2004 );3个精氨酸合成相关基因Car1、Agt1和Gbu1调控白念珠菌Candida albicans的生长和致病性(Schaeferet al. 2020 );天冬氨酸合成基因asd的缺失抑制白念珠菌的生长(Dahalet al. 2020 ).L-精氨酸是生防菌盾壳霉Coniothyrium minitans产孢所必需的,其衍生物一氧化氮可能介导其产孢功能(Gonget al. 2007 ).在植物病原菌中,尖孢镰孢菌甜瓜专化型F.oxysporum f. sp. melonis精氨酸合成基因ARG1缺失突变体(Namikiet al. 2001 )和灰霉菌精氨酸合成基因bcass1缺失突变体(Patelet al. 2010 )的致病性均减弱;甲硫氨酸合成基因FgMETB调控禾谷镰孢菌的孢子萌发、菌丝生长、DON毒素产生和致病性(Fuet al. 2013 );谷氨酸合成基因P5Cdh调控栗疫病菌Cryphonectria parasitica的致病性和产孢(Yaoet al. 2013 );甲硫氨酸合成基因MET13(Yanet al. 2013 )、赖氨酸合成基因MoLys2(Chenet al. 2014 )、苏氨酸合成基因MoIlv1(Duet al. 2014 )、精氨酸合成基因(MoARG1、MoARG5、MoARG6 和 MoARG7)(Zhanget al. 2015 )、亮氨酸合成基因(MoLEU1、MoLEU2、MoLEU4、LEU1、LEU2A和LEU4)(Queet al. 2020 ;Weiet al. 2020 )与稻瘟菌的生理特性和致病性密切相关.迄今,尚未有氨基酸与尖孢镰孢菌古巴专化型产孢、生长发育和致病性的相关报道. ...
... 通过对氨基酸合成通路93个差异表达基因的In silico分析,发现其中部分差异表达基因在其他真菌已得到鉴定,如FOIG_05525、FOIG_05468、FOIG_09550和FOIG_075934个基因的同源基因参与调控真菌亮氨酸的合成(Liebmannet al. 2004 ;Queet al. 2020 );FOIG_12215和FOIG_09392的同源基因参与调控尖孢镰孢菌甜瓜专化型精氨酸的合成(Namikiet al. 2001 );FOIG_12832和FOIG_03926的同源基因参与调控白念珠菌甘氨酸的合成(McNeilet al. 2000 );FOIG_05323的同源基因参与调控烟曲霉支链氨基酸的合成(Oliveret al. 2012 );FOIG_02315的同源基因参与调控白念珠菌天冬氨酸的合成(Dahalet al. 2020 );FOIG_01066、FOIG_01154、FOIG_10878、FOIG_02315、FOIG_07232、FOIG_07170、FOIG_03322、FOIG_05025、FOIG_10638、FOIG_07565、FOIG_0314711个基因的同源基因参与调控真菌的生长发育或产孢(Puyeskyet al. 1997 ;Baidyaroyet al. 2001 ;Rodakiet al. 2006 ;Dinget al. 2010 ;Kuret al. 2010 ;Gerkeet al. 2012 ;Fuet al. 2013 ;Duet al. 2014 ;Fernandezet al. 2014 ;Dahalet al. 2020 ;Schaeferet al. 2020 );FOIG_00393、FOIG_01066、FOIG_12215、FOIG_05468、FOIG_05352、FOIG_05323、FOIG_06701、FOIG_06470、FOIG_09550、FOIG_05525、FOIG_09392、FOIG_07410、FOIG_07232、FOIG_05897、FOIG_03295、FOIG_05025、FOIG_15020、FOIG_07593、FOIG_0756519个基因的同源基因参与调控真菌的致病性或毒素产生(Chenget al. 1999 ;Baidyaroyet al. 2001 ;Namikiet al. 2001 ;Liebmannet al. 2004 ;Seonget al. 2005 ;Dinget al. 2010 ;Kingsbury & McCusker 2010;Oliveret al. 2012 ;Fuet al. 2013 ;Yaoet al. 2013 ;Duet al. 2014 ;Liet al. 2018 ;Guoet al. 2019 ;Queet al. 2020 ;Schaeferet al. 2020 ;Weiet al. 2020 )(表1).由此说明,氨基酸合成通路不仅与尖孢镰孢菌古巴专化型的氨基酸合成和厚垣孢子形成相关,其有可能参与调控该病菌的致病性. ...
... ;Namikiet al. 2001 ;Liebmannet al. 2004 ;Seonget al. 2005 ;Dinget al. 2010 ;Kingsbury & McCusker 2010;Oliveret al. 2012 ;Fuet al. 2013 ;Yaoet al. 2013 ;Duet al. 2014 ;Liet al. 2018 ;Guoet al. 2019 ;Queet al. 2020 ;Schaeferet al. 2020 ;Weiet al. 2020 )(表1).由此说明,氨基酸合成通路不仅与尖孢镰孢菌古巴专化型的氨基酸合成和厚垣孢子形成相关,其有可能参与调控该病菌的致病性. ...
Characterization of the fusaric acid gene cluster in Fusarium fujikuroi
1
2014
... 尖孢镰孢菌古巴专化型的致病因子主要包括细胞壁降解酶、信号转导通路、毒素、sRNAs和致病相关基因.在病原菌和寄主互作过程中,病原菌通过分泌一系列细胞壁降解酶降解寄主植物的细胞壁,进而将多糖等营养物质从植物细胞内释放供病原菌生长,促进病原菌的侵入和定殖;多聚半乳糖醛酸酶已被鉴定为尖孢镰孢菌古巴专化型4号生理小种的致病因子(董章勇和王振中 2012).已有报道表明,FoSlt2 MAPK信号转导通路调控尖孢镰孢菌古巴专化型的生理特性和致病性(Dinget al. 2015 ;Dinget al. 2018a ;丁兆建等 2019b;Dinget al. 2020 );cAMP-PKA信号通路G蛋白亚基FGA2和FGB1参与调控尖孢镰孢菌古巴专化型的生长发育、产孢、耐热性和致病性(Guoet al. 2016 ).镰孢菌酸(fusaric acid,FA)是镰孢菌产生的非寄主专化性毒素,对动物和人类具有中低毒性,但对植物有较高的毒性,可引起多种作物的萎蔫病和根腐病害(Brownet al. 2012 ;Niehauset al. 2014 ).国内外已报道尖孢镰孢菌古巴专化型的多个致萎相关毒素,包括FA、白僵菌素和伏马菌素B1(许文耀等 2004;陈石等 2011;李春雨等 2011;Ding et al. 2018b ;Portal et al. 2018 ).关于植物病原真菌致病相关sRNAs报道较多,灰霉菌Botrytis cinerea、大丽轮枝菌Verticillium dahlia、小麦柄锈菌Puccinia striiformis f. sp. tritici和卵菌Oomycetes产生的sRNAs效应子可跨界传输到寄主细胞中,通过沉默寄主相关免疫基因,抑制寄主植物的免疫反应(Weiberget al. 2013 ;Wanget al. 2016 ;Wanget al. 2017 );关于尖孢镰孢菌古巴专化型sRNAs的研究处于起步阶段,李敏慧等(2019)发现用该病菌接种香蕉苗根部24h后,病原菌编码Dicer 和Argonaute的基因表达显著升高,sRNA测序结果也表明,大量milRNA显著高表达.也有多个相关致病基因得到鉴定,如活性氧迸发转录因子Foatf1(Qiet al. 2013 )、编码α-1,6-甘露糖转移酶的基因FoOCH1(Liet al. 2014 )、微管蛋白β1和β2(刘远征等 2018a,2018b)和Ⅴ型肌球蛋白Myosin5的协作蛋白smy1(何壮等 2019).尽管该菌的致病机理研究已取得了显著进展,但其致病机制仍不清晰,尤其对作为初侵染源的厚垣孢子的研究也尚未引起足够的重视. ...
FoSTUA, encoding a basic helix-loop-helix protein, differentially regulates development of three kinds of asexual spores, macroconidia, microconidia, and chlamydospores, in the fungal plant pathogen Fusarium oxysporum
1
2004
... 香蕉枯萎病是由尖孢镰孢菌古巴专化型 Fusarium oxysporum f. sp. cubense侵染引起的一种土传真菌病害,现已成为热带和亚热带地区香蕉产业的重大威胁(Ploetz 2006).根据对不同香蕉品种的致病力差异分析,该病菌被分为3个生理小种(1、2和4号生理小种),其中1号生理小种侵染大密哈(Gros Michel,AAA)和基因型为AAB的香蕉品种;2号生理小种侵染棱香蕉(Bluggoe,AAB);4号生理小种几乎侵染所有的香蕉品种,给种植户造成了严重的经济损失(Su et al. 1986).该病菌产生3种类型的孢子,分别为大型分生孢子、小型分生孢子和厚垣孢子;其中大型分生孢子和小型分生孢子是次级侵染源,多形成于香蕉寄主根部和假茎的表面;厚垣孢子是一种厚壁休眠孢子,是初级侵染源,其可在土壤中存活多年甚至10年以上.当厚垣孢子受到香蕉根部分泌物的信号刺激后,会萌发形成菌丝,侵染香蕉寄主,导致发生香蕉枯萎病(Katan et al. 1997;Ohara & Tsuge 2004;丁兆建等2019a). ...
The Aspergillus fumigatusdihydroxyacid dehydratase Ilv3A/IlvC is required for full virulence
2
2012
... 通过对氨基酸合成通路93个差异表达基因的In silico分析,发现其中部分差异表达基因在其他真菌已得到鉴定,如FOIG_05525、FOIG_05468、FOIG_09550和FOIG_075934个基因的同源基因参与调控真菌亮氨酸的合成(Liebmannet al. 2004 ;Queet al. 2020 );FOIG_12215和FOIG_09392的同源基因参与调控尖孢镰孢菌甜瓜专化型精氨酸的合成(Namikiet al. 2001 );FOIG_12832和FOIG_03926的同源基因参与调控白念珠菌甘氨酸的合成(McNeilet al. 2000 );FOIG_05323的同源基因参与调控烟曲霉支链氨基酸的合成(Oliveret al. 2012 );FOIG_02315的同源基因参与调控白念珠菌天冬氨酸的合成(Dahalet al. 2020 );FOIG_01066、FOIG_01154、FOIG_10878、FOIG_02315、FOIG_07232、FOIG_07170、FOIG_03322、FOIG_05025、FOIG_10638、FOIG_07565、FOIG_0314711个基因的同源基因参与调控真菌的生长发育或产孢(Puyeskyet al. 1997 ;Baidyaroyet al. 2001 ;Rodakiet al. 2006 ;Dinget al. 2010 ;Kuret al. 2010 ;Gerkeet al. 2012 ;Fuet al. 2013 ;Duet al. 2014 ;Fernandezet al. 2014 ;Dahalet al. 2020 ;Schaeferet al. 2020 );FOIG_00393、FOIG_01066、FOIG_12215、FOIG_05468、FOIG_05352、FOIG_05323、FOIG_06701、FOIG_06470、FOIG_09550、FOIG_05525、FOIG_09392、FOIG_07410、FOIG_07232、FOIG_05897、FOIG_03295、FOIG_05025、FOIG_15020、FOIG_07593、FOIG_0756519个基因的同源基因参与调控真菌的致病性或毒素产生(Chenget al. 1999 ;Baidyaroyet al. 2001 ;Namikiet al. 2001 ;Liebmannet al. 2004 ;Seonget al. 2005 ;Dinget al. 2010 ;Kingsbury & McCusker 2010;Oliveret al. 2012 ;Fuet al. 2013 ;Yaoet al. 2013 ;Duet al. 2014 ;Liet al. 2018 ;Guoet al. 2019 ;Queet al. 2020 ;Schaeferet al. 2020 ;Weiet al. 2020 )(表1).由此说明,氨基酸合成通路不仅与尖孢镰孢菌古巴专化型的氨基酸合成和厚垣孢子形成相关,其有可能参与调控该病菌的致病性. ...
... ;Oliveret al. 2012 ;Fuet al. 2013 ;Yaoet al. 2013 ;Duet al. 2014 ;Liet al. 2018 ;Guoet al. 2019 ;Queet al. 2020 ;Schaeferet al. 2020 ;Weiet al. 2020 )(表1).由此说明,氨基酸合成通路不仅与尖孢镰孢菌古巴专化型的氨基酸合成和厚垣孢子形成相关,其有可能参与调控该病菌的致病性. ...
Inadvertent gene silencing of argininosuccinate synthase (bcass1) inBotrytis cinerea by the pLOB1 vector system
1
2010
... 氨基酸是重要的有机化合物,其在蛋白质合成、细胞生长、发育和能量产生等多个生物学过程中起着重要作用.已有研究证明氨基酸代谢与真菌的产孢、生长和致病性相关.在人类致病菌中,赖氨酸合成基因lysF调控烟曲霉Aspergillus fumigatus的致病性(Liebmannet al. 2004 );3个精氨酸合成相关基因Car1、Agt1和Gbu1调控白念珠菌Candida albicans的生长和致病性(Schaeferet al. 2020 );天冬氨酸合成基因asd的缺失抑制白念珠菌的生长(Dahalet al. 2020 ).L-精氨酸是生防菌盾壳霉Coniothyrium minitans产孢所必需的,其衍生物一氧化氮可能介导其产孢功能(Gonget al. 2007 ).在植物病原菌中,尖孢镰孢菌甜瓜专化型F.oxysporum f. sp. melonis精氨酸合成基因ARG1缺失突变体(Namikiet al. 2001 )和灰霉菌精氨酸合成基因bcass1缺失突变体(Patelet al. 2010 )的致病性均减弱;甲硫氨酸合成基因FgMETB调控禾谷镰孢菌的孢子萌发、菌丝生长、DON毒素产生和致病性(Fuet al. 2013 );谷氨酸合成基因P5Cdh调控栗疫病菌Cryphonectria parasitica的致病性和产孢(Yaoet al. 2013 );甲硫氨酸合成基因MET13(Yanet al. 2013 )、赖氨酸合成基因MoLys2(Chenet al. 2014 )、苏氨酸合成基因MoIlv1(Duet al. 2014 )、精氨酸合成基因(MoARG1、MoARG5、MoARG6 和 MoARG7)(Zhanget al. 2015 )、亮氨酸合成基因(MoLEU1、MoLEU2、MoLEU4、LEU1、LEU2A和LEU4)(Queet al. 2020 ;Weiet al. 2020 )与稻瘟菌的生理特性和致病性密切相关.迄今,尚未有氨基酸与尖孢镰孢菌古巴专化型产孢、生长发育和致病性的相关报道. ...
Fusarium wilt of banana is caused by several pathogens referred to as Fusarium oxysporum f. sp. cubense
1
2006
... 香蕉枯萎病是由尖孢镰孢菌古巴专化型 Fusarium oxysporum f. sp. cubense侵染引起的一种土传真菌病害,现已成为热带和亚热带地区香蕉产业的重大威胁(Ploetz 2006).根据对不同香蕉品种的致病力差异分析,该病菌被分为3个生理小种(1、2和4号生理小种),其中1号生理小种侵染大密哈(Gros Michel,AAA)和基因型为AAB的香蕉品种;2号生理小种侵染棱香蕉(Bluggoe,AAB);4号生理小种几乎侵染所有的香蕉品种,给种植户造成了严重的经济损失(Su et al. 1986).该病菌产生3种类型的孢子,分别为大型分生孢子、小型分生孢子和厚垣孢子;其中大型分生孢子和小型分生孢子是次级侵染源,多形成于香蕉寄主根部和假茎的表面;厚垣孢子是一种厚壁休眠孢子,是初级侵染源,其可在土壤中存活多年甚至10年以上.当厚垣孢子受到香蕉根部分泌物的信号刺激后,会萌发形成菌丝,侵染香蕉寄主,导致发生香蕉枯萎病(Katan et al. 1997;Ohara & Tsuge 2004;丁兆建等2019a). ...
Nonspecific toxins as components of a host-specific culture filtrate from Fusarium oxysporum f. sp. cubense race 1
1
2018
... 尖孢镰孢菌古巴专化型的致病因子主要包括细胞壁降解酶、信号转导通路、毒素、sRNAs和致病相关基因.在病原菌和寄主互作过程中,病原菌通过分泌一系列细胞壁降解酶降解寄主植物的细胞壁,进而将多糖等营养物质从植物细胞内释放供病原菌生长,促进病原菌的侵入和定殖;多聚半乳糖醛酸酶已被鉴定为尖孢镰孢菌古巴专化型4号生理小种的致病因子(董章勇和王振中 2012).已有报道表明,FoSlt2 MAPK信号转导通路调控尖孢镰孢菌古巴专化型的生理特性和致病性(Dinget al. 2015 ;Dinget al. 2018a ;丁兆建等 2019b;Dinget al. 2020 );cAMP-PKA信号通路G蛋白亚基FGA2和FGB1参与调控尖孢镰孢菌古巴专化型的生长发育、产孢、耐热性和致病性(Guoet al. 2016 ).镰孢菌酸(fusaric acid,FA)是镰孢菌产生的非寄主专化性毒素,对动物和人类具有中低毒性,但对植物有较高的毒性,可引起多种作物的萎蔫病和根腐病害(Brownet al. 2012 ;Niehauset al. 2014 ).国内外已报道尖孢镰孢菌古巴专化型的多个致萎相关毒素,包括FA、白僵菌素和伏马菌素B1(许文耀等 2004;陈石等 2011;李春雨等 2011;Ding et al. 2018b ;Portal et al. 2018 ).关于植物病原真菌致病相关sRNAs报道较多,灰霉菌Botrytis cinerea、大丽轮枝菌Verticillium dahlia、小麦柄锈菌Puccinia striiformis f. sp. tritici和卵菌Oomycetes产生的sRNAs效应子可跨界传输到寄主细胞中,通过沉默寄主相关免疫基因,抑制寄主植物的免疫反应(Weiberget al. 2013 ;Wanget al. 2016 ;Wanget al. 2017 );关于尖孢镰孢菌古巴专化型sRNAs的研究处于起步阶段,李敏慧等(2019)发现用该病菌接种香蕉苗根部24h后,病原菌编码Dicer 和Argonaute的基因表达显著升高,sRNA测序结果也表明,大量milRNA显著高表达.也有多个相关致病基因得到鉴定,如活性氧迸发转录因子Foatf1(Qiet al. 2013 )、编码α-1,6-甘露糖转移酶的基因FoOCH1(Liet al. 2014 )、微管蛋白β1和β2(刘远征等 2018a,2018b)和Ⅴ型肌球蛋白Myosin5的协作蛋白smy1(何壮等 2019).尽管该菌的致病机理研究已取得了显著进展,但其致病机制仍不清晰,尤其对作为初侵染源的厚垣孢子的研究也尚未引起足够的重视. ...
Glyceraldehyde-3- phosphate dehydrogenase expression in Trichoderma harzianum is repressed during conidiation and mycoparasitism
1
1997
... 通过对氨基酸合成通路93个差异表达基因的In silico分析,发现其中部分差异表达基因在其他真菌已得到鉴定,如FOIG_05525、FOIG_05468、FOIG_09550和FOIG_075934个基因的同源基因参与调控真菌亮氨酸的合成(Liebmannet al. 2004 ;Queet al. 2020 );FOIG_12215和FOIG_09392的同源基因参与调控尖孢镰孢菌甜瓜专化型精氨酸的合成(Namikiet al. 2001 );FOIG_12832和FOIG_03926的同源基因参与调控白念珠菌甘氨酸的合成(McNeilet al. 2000 );FOIG_05323的同源基因参与调控烟曲霉支链氨基酸的合成(Oliveret al. 2012 );FOIG_02315的同源基因参与调控白念珠菌天冬氨酸的合成(Dahalet al. 2020 );FOIG_01066、FOIG_01154、FOIG_10878、FOIG_02315、FOIG_07232、FOIG_07170、FOIG_03322、FOIG_05025、FOIG_10638、FOIG_07565、FOIG_0314711个基因的同源基因参与调控真菌的生长发育或产孢(Puyeskyet al. 1997 ;Baidyaroyet al. 2001 ;Rodakiet al. 2006 ;Dinget al. 2010 ;Kuret al. 2010 ;Gerkeet al. 2012 ;Fuet al. 2013 ;Duet al. 2014 ;Fernandezet al. 2014 ;Dahalet al. 2020 ;Schaeferet al. 2020 );FOIG_00393、FOIG_01066、FOIG_12215、FOIG_05468、FOIG_05352、FOIG_05323、FOIG_06701、FOIG_06470、FOIG_09550、FOIG_05525、FOIG_09392、FOIG_07410、FOIG_07232、FOIG_05897、FOIG_03295、FOIG_05025、FOIG_15020、FOIG_07593、FOIG_0756519个基因的同源基因参与调控真菌的致病性或毒素产生(Chenget al. 1999 ;Baidyaroyet al. 2001 ;Namikiet al. 2001 ;Liebmannet al. 2004 ;Seonget al. 2005 ;Dinget al. 2010 ;Kingsbury & McCusker 2010;Oliveret al. 2012 ;Fuet al. 2013 ;Yaoet al. 2013 ;Duet al. 2014 ;Liet al. 2018 ;Guoet al. 2019 ;Queet al. 2020 ;Schaeferet al. 2020 ;Weiet al. 2020 )(表1).由此说明,氨基酸合成通路不仅与尖孢镰孢菌古巴专化型的氨基酸合成和厚垣孢子形成相关,其有可能参与调控该病菌的致病性. ...
Foatf1, a bZIP transcription factor of Fusarium oxysporum f. sp. cubense, is involved in pathogenesis by regulating the oxidative stress responses of cavendish banana (Musa spp.)
1
2013
... 尖孢镰孢菌古巴专化型的致病因子主要包括细胞壁降解酶、信号转导通路、毒素、sRNAs和致病相关基因.在病原菌和寄主互作过程中,病原菌通过分泌一系列细胞壁降解酶降解寄主植物的细胞壁,进而将多糖等营养物质从植物细胞内释放供病原菌生长,促进病原菌的侵入和定殖;多聚半乳糖醛酸酶已被鉴定为尖孢镰孢菌古巴专化型4号生理小种的致病因子(董章勇和王振中 2012).已有报道表明,FoSlt2 MAPK信号转导通路调控尖孢镰孢菌古巴专化型的生理特性和致病性(Dinget al. 2015 ;Dinget al. 2018a ;丁兆建等 2019b;Dinget al. 2020 );cAMP-PKA信号通路G蛋白亚基FGA2和FGB1参与调控尖孢镰孢菌古巴专化型的生长发育、产孢、耐热性和致病性(Guoet al. 2016 ).镰孢菌酸(fusaric acid,FA)是镰孢菌产生的非寄主专化性毒素,对动物和人类具有中低毒性,但对植物有较高的毒性,可引起多种作物的萎蔫病和根腐病害(Brownet al. 2012 ;Niehauset al. 2014 ).国内外已报道尖孢镰孢菌古巴专化型的多个致萎相关毒素,包括FA、白僵菌素和伏马菌素B1(许文耀等 2004;陈石等 2011;李春雨等 2011;Ding et al. 2018b ;Portal et al. 2018 ).关于植物病原真菌致病相关sRNAs报道较多,灰霉菌Botrytis cinerea、大丽轮枝菌Verticillium dahlia、小麦柄锈菌Puccinia striiformis f. sp. tritici和卵菌Oomycetes产生的sRNAs效应子可跨界传输到寄主细胞中,通过沉默寄主相关免疫基因,抑制寄主植物的免疫反应(Weiberget al. 2013 ;Wanget al. 2016 ;Wanget al. 2017 );关于尖孢镰孢菌古巴专化型sRNAs的研究处于起步阶段,李敏慧等(2019)发现用该病菌接种香蕉苗根部24h后,病原菌编码Dicer 和Argonaute的基因表达显著升高,sRNA测序结果也表明,大量milRNA显著高表达.也有多个相关致病基因得到鉴定,如活性氧迸发转录因子Foatf1(Qiet al. 2013 )、编码α-1,6-甘露糖转移酶的基因FoOCH1(Liet al. 2014 )、微管蛋白β1和β2(刘远征等 2018a,2018b)和Ⅴ型肌球蛋白Myosin5的协作蛋白smy1(何壮等 2019).尽管该菌的致病机理研究已取得了显著进展,但其致病机制仍不清晰,尤其对作为初侵染源的厚垣孢子的研究也尚未引起足够的重视. ...
Leucine biosynthesis is required for infection-related morphogenesis and pathogenicity in the rice blast fungus Magnaporthe oryzae
4
2020
... 氨基酸是重要的有机化合物,其在蛋白质合成、细胞生长、发育和能量产生等多个生物学过程中起着重要作用.已有研究证明氨基酸代谢与真菌的产孢、生长和致病性相关.在人类致病菌中,赖氨酸合成基因lysF调控烟曲霉Aspergillus fumigatus的致病性(Liebmannet al. 2004 );3个精氨酸合成相关基因Car1、Agt1和Gbu1调控白念珠菌Candida albicans的生长和致病性(Schaeferet al. 2020 );天冬氨酸合成基因asd的缺失抑制白念珠菌的生长(Dahalet al. 2020 ).L-精氨酸是生防菌盾壳霉Coniothyrium minitans产孢所必需的,其衍生物一氧化氮可能介导其产孢功能(Gonget al. 2007 ).在植物病原菌中,尖孢镰孢菌甜瓜专化型F.oxysporum f. sp. melonis精氨酸合成基因ARG1缺失突变体(Namikiet al. 2001 )和灰霉菌精氨酸合成基因bcass1缺失突变体(Patelet al. 2010 )的致病性均减弱;甲硫氨酸合成基因FgMETB调控禾谷镰孢菌的孢子萌发、菌丝生长、DON毒素产生和致病性(Fuet al. 2013 );谷氨酸合成基因P5Cdh调控栗疫病菌Cryphonectria parasitica的致病性和产孢(Yaoet al. 2013 );甲硫氨酸合成基因MET13(Yanet al. 2013 )、赖氨酸合成基因MoLys2(Chenet al. 2014 )、苏氨酸合成基因MoIlv1(Duet al. 2014 )、精氨酸合成基因(MoARG1、MoARG5、MoARG6 和 MoARG7)(Zhanget al. 2015 )、亮氨酸合成基因(MoLEU1、MoLEU2、MoLEU4、LEU1、LEU2A和LEU4)(Queet al. 2020 ;Weiet al. 2020 )与稻瘟菌的生理特性和致病性密切相关.迄今,尚未有氨基酸与尖孢镰孢菌古巴专化型产孢、生长发育和致病性的相关报道. ...
... 通过对氨基酸合成通路93个差异表达基因的In silico分析,发现其中部分差异表达基因在其他真菌已得到鉴定,如FOIG_05525、FOIG_05468、FOIG_09550和FOIG_075934个基因的同源基因参与调控真菌亮氨酸的合成(Liebmannet al. 2004 ;Queet al. 2020 );FOIG_12215和FOIG_09392的同源基因参与调控尖孢镰孢菌甜瓜专化型精氨酸的合成(Namikiet al. 2001 );FOIG_12832和FOIG_03926的同源基因参与调控白念珠菌甘氨酸的合成(McNeilet al. 2000 );FOIG_05323的同源基因参与调控烟曲霉支链氨基酸的合成(Oliveret al. 2012 );FOIG_02315的同源基因参与调控白念珠菌天冬氨酸的合成(Dahalet al. 2020 );FOIG_01066、FOIG_01154、FOIG_10878、FOIG_02315、FOIG_07232、FOIG_07170、FOIG_03322、FOIG_05025、FOIG_10638、FOIG_07565、FOIG_0314711个基因的同源基因参与调控真菌的生长发育或产孢(Puyeskyet al. 1997 ;Baidyaroyet al. 2001 ;Rodakiet al. 2006 ;Dinget al. 2010 ;Kuret al. 2010 ;Gerkeet al. 2012 ;Fuet al. 2013 ;Duet al. 2014 ;Fernandezet al. 2014 ;Dahalet al. 2020 ;Schaeferet al. 2020 );FOIG_00393、FOIG_01066、FOIG_12215、FOIG_05468、FOIG_05352、FOIG_05323、FOIG_06701、FOIG_06470、FOIG_09550、FOIG_05525、FOIG_09392、FOIG_07410、FOIG_07232、FOIG_05897、FOIG_03295、FOIG_05025、FOIG_15020、FOIG_07593、FOIG_0756519个基因的同源基因参与调控真菌的致病性或毒素产生(Chenget al. 1999 ;Baidyaroyet al. 2001 ;Namikiet al. 2001 ;Liebmannet al. 2004 ;Seonget al. 2005 ;Dinget al. 2010 ;Kingsbury & McCusker 2010;Oliveret al. 2012 ;Fuet al. 2013 ;Yaoet al. 2013 ;Duet al. 2014 ;Liet al. 2018 ;Guoet al. 2019 ;Queet al. 2020 ;Schaeferet al. 2020 ;Weiet al. 2020 )(表1).由此说明,氨基酸合成通路不仅与尖孢镰孢菌古巴专化型的氨基酸合成和厚垣孢子形成相关,其有可能参与调控该病菌的致病性. ...
... ;Queet al. 2020 ;Schaeferet al. 2020 ;Weiet al. 2020 )(表1).由此说明,氨基酸合成通路不仅与尖孢镰孢菌古巴专化型的氨基酸合成和厚垣孢子形成相关,其有可能参与调控该病菌的致病性. ...
... 氨基酸合成通路通过调控氨基酸的合成,影响尖孢镰孢菌古巴专化型厚垣孢子的形成.前人的研究表明,氨基酸合成基因的敲除能导致氨基酸的合成缺失,同时影响真菌生长或产孢,如白念珠菌精氨酸合成基因 Car1、Agt1和Gbu1(Schaeferet al. 2020 )及天冬氨酸合成基因asd(Dahalet al. 2020 ),禾谷镰孢菌甲硫氨酸合成基因FgMETB(Fuet al. 2013 )、栗疫病菌谷氨酸合成基因 P5Cdh(Yaoet al. 2013 )、稻瘟菌甲硫氨酸合成基因MET13(Yanet al. 2013 )、赖氨酸合成基因MoLys2(Chenet al. 2014 )、苏氨酸合成基因MoIlv1(Duet al. 2014 )、精氨酸合成基因(MoARG1、MoARG5、MoARG6和MoARG7)(Zhanget al. 2015 )、亮氨酸合成基因(MoLEU1、MoLEU2、MoLEU4、LEU1、LEU2A 和LEU4)(Queet al. 2020 ;Weiet al. 2020 ).值得指出的是,这些氨基酸合成基因的同源基因的表达水平在尖孢镰孢菌古巴专化型厚垣孢子形成过程中均发生了变化.此外,本研究发现外源甘氨酸抑制尖孢镰孢菌古巴专化型厚垣孢子的诱导形成.这些数据确认了氨基酸合成基因与尖孢镰孢菌古巴专化型厚垣孢子形成存在联系,氨基酸合成通路可通过调控氨基酸合成基因的转录,进而影响尖孢镰孢菌古巴专化型厚垣孢子的形成. ...
Observations on chlamydospore production by Fusarium in a two-salt solution
1
1970
... 氨基酸如何影响真菌产孢、生长或者抑制尖孢镰孢菌古巴专化型厚垣孢子形成呢?真菌为适应不同或者变化的环境,会尽可能广泛地选择营养来源.有些真菌种类利用氨基酸的降解产物作为唯一氮源,有的则利用其作为唯一碳源,有的则两者兼有之(Rzadet al. 2018 ),如白念珠菌、新型隐球菌Cryptococcus neoformans和烟曲霉利用赖氨酸作为唯一氮源,酿酒酵母则利用赖氨酸作为唯一的碳源而非唯一氮源(Kinzelet al. 1983 ).已有报道表明,碳源的浓度与真菌厚垣孢子的形成相关,高浓度的葡萄糖抑制白色念珠菌和镰孢菌厚垣孢子的形成,缺乏或者低浓度的葡萄糖可促使镰孢菌厚垣孢子的形成(Qureshi & Page 1970;Griffin 1976;Mandal & Chaudhuri 2013;Bottcheret al. 2016 ).前期的研究发现,作为碳源的N-乙酰葡糖胺浓度达到20g/L时,尖孢镰孢菌古巴专化型的菌丝不产生厚垣孢子;当N-乙酰葡糖胺缺乏时,其菌丝可产生大量厚垣孢子(丁兆建等 2019a).由此推测,外源甘氨酸的添加导致碳源的浓度升高,从而抑制尖孢镰孢菌古巴专化型厚垣孢子的诱导形成. ...
Effects of depleting the essential central metabolic enzyme fructose-1,6-bisphosphate aldolase on the growth and viability ofCandida albicans: implications for antifungal drug target discovery
1
2006
... 通过对氨基酸合成通路93个差异表达基因的In silico分析,发现其中部分差异表达基因在其他真菌已得到鉴定,如FOIG_05525、FOIG_05468、FOIG_09550和FOIG_075934个基因的同源基因参与调控真菌亮氨酸的合成(Liebmannet al. 2004 ;Queet al. 2020 );FOIG_12215和FOIG_09392的同源基因参与调控尖孢镰孢菌甜瓜专化型精氨酸的合成(Namikiet al. 2001 );FOIG_12832和FOIG_03926的同源基因参与调控白念珠菌甘氨酸的合成(McNeilet al. 2000 );FOIG_05323的同源基因参与调控烟曲霉支链氨基酸的合成(Oliveret al. 2012 );FOIG_02315的同源基因参与调控白念珠菌天冬氨酸的合成(Dahalet al. 2020 );FOIG_01066、FOIG_01154、FOIG_10878、FOIG_02315、FOIG_07232、FOIG_07170、FOIG_03322、FOIG_05025、FOIG_10638、FOIG_07565、FOIG_0314711个基因的同源基因参与调控真菌的生长发育或产孢(Puyeskyet al. 1997 ;Baidyaroyet al. 2001 ;Rodakiet al. 2006 ;Dinget al. 2010 ;Kuret al. 2010 ;Gerkeet al. 2012 ;Fuet al. 2013 ;Duet al. 2014 ;Fernandezet al. 2014 ;Dahalet al. 2020 ;Schaeferet al. 2020 );FOIG_00393、FOIG_01066、FOIG_12215、FOIG_05468、FOIG_05352、FOIG_05323、FOIG_06701、FOIG_06470、FOIG_09550、FOIG_05525、FOIG_09392、FOIG_07410、FOIG_07232、FOIG_05897、FOIG_03295、FOIG_05025、FOIG_15020、FOIG_07593、FOIG_0756519个基因的同源基因参与调控真菌的致病性或毒素产生(Chenget al. 1999 ;Baidyaroyet al. 2001 ;Namikiet al. 2001 ;Liebmannet al. 2004 ;Seonget al. 2005 ;Dinget al. 2010 ;Kingsbury & McCusker 2010;Oliveret al. 2012 ;Fuet al. 2013 ;Yaoet al. 2013 ;Duet al. 2014 ;Liet al. 2018 ;Guoet al. 2019 ;Queet al. 2020 ;Schaeferet al. 2020 ;Weiet al. 2020 )(表1).由此说明,氨基酸合成通路不仅与尖孢镰孢菌古巴专化型的氨基酸合成和厚垣孢子形成相关,其有可能参与调控该病菌的致病性. ...
Versatility of putative aromatic aminotransferases from Candida albicans
1
2018
... 氨基酸如何影响真菌产孢、生长或者抑制尖孢镰孢菌古巴专化型厚垣孢子形成呢?真菌为适应不同或者变化的环境,会尽可能广泛地选择营养来源.有些真菌种类利用氨基酸的降解产物作为唯一氮源,有的则利用其作为唯一碳源,有的则两者兼有之(Rzadet al. 2018 ),如白念珠菌、新型隐球菌Cryptococcus neoformans和烟曲霉利用赖氨酸作为唯一氮源,酿酒酵母则利用赖氨酸作为唯一的碳源而非唯一氮源(Kinzelet al. 1983 ).已有报道表明,碳源的浓度与真菌厚垣孢子的形成相关,高浓度的葡萄糖抑制白色念珠菌和镰孢菌厚垣孢子的形成,缺乏或者低浓度的葡萄糖可促使镰孢菌厚垣孢子的形成(Qureshi & Page 1970;Griffin 1976;Mandal & Chaudhuri 2013;Bottcheret al. 2016 ).前期的研究发现,作为碳源的N-乙酰葡糖胺浓度达到20g/L时,尖孢镰孢菌古巴专化型的菌丝不产生厚垣孢子;当N-乙酰葡糖胺缺乏时,其菌丝可产生大量厚垣孢子(丁兆建等 2019a).由此推测,外源甘氨酸的添加导致碳源的浓度升高,从而抑制尖孢镰孢菌古巴专化型厚垣孢子的诱导形成. ...
Three related enzymes in Candida albicans achieve arginine- and agmatine-dependent metabolism that is essential for growth and fungal virulence
4
2020
... 氨基酸是重要的有机化合物,其在蛋白质合成、细胞生长、发育和能量产生等多个生物学过程中起着重要作用.已有研究证明氨基酸代谢与真菌的产孢、生长和致病性相关.在人类致病菌中,赖氨酸合成基因lysF调控烟曲霉Aspergillus fumigatus的致病性(Liebmannet al. 2004 );3个精氨酸合成相关基因Car1、Agt1和Gbu1调控白念珠菌Candida albicans的生长和致病性(Schaeferet al. 2020 );天冬氨酸合成基因asd的缺失抑制白念珠菌的生长(Dahalet al. 2020 ).L-精氨酸是生防菌盾壳霉Coniothyrium minitans产孢所必需的,其衍生物一氧化氮可能介导其产孢功能(Gonget al. 2007 ).在植物病原菌中,尖孢镰孢菌甜瓜专化型F.oxysporum f. sp. melonis精氨酸合成基因ARG1缺失突变体(Namikiet al. 2001 )和灰霉菌精氨酸合成基因bcass1缺失突变体(Patelet al. 2010 )的致病性均减弱;甲硫氨酸合成基因FgMETB调控禾谷镰孢菌的孢子萌发、菌丝生长、DON毒素产生和致病性(Fuet al. 2013 );谷氨酸合成基因P5Cdh调控栗疫病菌Cryphonectria parasitica的致病性和产孢(Yaoet al. 2013 );甲硫氨酸合成基因MET13(Yanet al. 2013 )、赖氨酸合成基因MoLys2(Chenet al. 2014 )、苏氨酸合成基因MoIlv1(Duet al. 2014 )、精氨酸合成基因(MoARG1、MoARG5、MoARG6 和 MoARG7)(Zhanget al. 2015 )、亮氨酸合成基因(MoLEU1、MoLEU2、MoLEU4、LEU1、LEU2A和LEU4)(Queet al. 2020 ;Weiet al. 2020 )与稻瘟菌的生理特性和致病性密切相关.迄今,尚未有氨基酸与尖孢镰孢菌古巴专化型产孢、生长发育和致病性的相关报道. ...
... 通过对氨基酸合成通路93个差异表达基因的In silico分析,发现其中部分差异表达基因在其他真菌已得到鉴定,如FOIG_05525、FOIG_05468、FOIG_09550和FOIG_075934个基因的同源基因参与调控真菌亮氨酸的合成(Liebmannet al. 2004 ;Queet al. 2020 );FOIG_12215和FOIG_09392的同源基因参与调控尖孢镰孢菌甜瓜专化型精氨酸的合成(Namikiet al. 2001 );FOIG_12832和FOIG_03926的同源基因参与调控白念珠菌甘氨酸的合成(McNeilet al. 2000 );FOIG_05323的同源基因参与调控烟曲霉支链氨基酸的合成(Oliveret al. 2012 );FOIG_02315的同源基因参与调控白念珠菌天冬氨酸的合成(Dahalet al. 2020 );FOIG_01066、FOIG_01154、FOIG_10878、FOIG_02315、FOIG_07232、FOIG_07170、FOIG_03322、FOIG_05025、FOIG_10638、FOIG_07565、FOIG_0314711个基因的同源基因参与调控真菌的生长发育或产孢(Puyeskyet al. 1997 ;Baidyaroyet al. 2001 ;Rodakiet al. 2006 ;Dinget al. 2010 ;Kuret al. 2010 ;Gerkeet al. 2012 ;Fuet al. 2013 ;Duet al. 2014 ;Fernandezet al. 2014 ;Dahalet al. 2020 ;Schaeferet al. 2020 );FOIG_00393、FOIG_01066、FOIG_12215、FOIG_05468、FOIG_05352、FOIG_05323、FOIG_06701、FOIG_06470、FOIG_09550、FOIG_05525、FOIG_09392、FOIG_07410、FOIG_07232、FOIG_05897、FOIG_03295、FOIG_05025、FOIG_15020、FOIG_07593、FOIG_0756519个基因的同源基因参与调控真菌的致病性或毒素产生(Chenget al. 1999 ;Baidyaroyet al. 2001 ;Namikiet al. 2001 ;Liebmannet al. 2004 ;Seonget al. 2005 ;Dinget al. 2010 ;Kingsbury & McCusker 2010;Oliveret al. 2012 ;Fuet al. 2013 ;Yaoet al. 2013 ;Duet al. 2014 ;Liet al. 2018 ;Guoet al. 2019 ;Queet al. 2020 ;Schaeferet al. 2020 ;Weiet al. 2020 )(表1).由此说明,氨基酸合成通路不仅与尖孢镰孢菌古巴专化型的氨基酸合成和厚垣孢子形成相关,其有可能参与调控该病菌的致病性. ...
... ;Schaeferet al. 2020 ;Weiet al. 2020 )(表1).由此说明,氨基酸合成通路不仅与尖孢镰孢菌古巴专化型的氨基酸合成和厚垣孢子形成相关,其有可能参与调控该病菌的致病性. ...
... 氨基酸合成通路通过调控氨基酸的合成,影响尖孢镰孢菌古巴专化型厚垣孢子的形成.前人的研究表明,氨基酸合成基因的敲除能导致氨基酸的合成缺失,同时影响真菌生长或产孢,如白念珠菌精氨酸合成基因 Car1、Agt1和Gbu1(Schaeferet al. 2020 )及天冬氨酸合成基因asd(Dahalet al. 2020 ),禾谷镰孢菌甲硫氨酸合成基因FgMETB(Fuet al. 2013 )、栗疫病菌谷氨酸合成基因 P5Cdh(Yaoet al. 2013 )、稻瘟菌甲硫氨酸合成基因MET13(Yanet al. 2013 )、赖氨酸合成基因MoLys2(Chenet al. 2014 )、苏氨酸合成基因MoIlv1(Duet al. 2014 )、精氨酸合成基因(MoARG1、MoARG5、MoARG6和MoARG7)(Zhanget al. 2015 )、亮氨酸合成基因(MoLEU1、MoLEU2、MoLEU4、LEU1、LEU2A 和LEU4)(Queet al. 2020 ;Weiet al. 2020 ).值得指出的是,这些氨基酸合成基因的同源基因的表达水平在尖孢镰孢菌古巴专化型厚垣孢子形成过程中均发生了变化.此外,本研究发现外源甘氨酸抑制尖孢镰孢菌古巴专化型厚垣孢子的诱导形成.这些数据确认了氨基酸合成基因与尖孢镰孢菌古巴专化型厚垣孢子形成存在联系,氨基酸合成通路可通过调控氨基酸合成基因的转录,进而影响尖孢镰孢菌古巴专化型厚垣孢子的形成. ...
Random insertional mutagenesis identifies genes associated with virulence in the wheat scab fungus Fusarium graminearum
1
2005
... 通过对氨基酸合成通路93个差异表达基因的In silico分析,发现其中部分差异表达基因在其他真菌已得到鉴定,如FOIG_05525、FOIG_05468、FOIG_09550和FOIG_075934个基因的同源基因参与调控真菌亮氨酸的合成(Liebmannet al. 2004 ;Queet al. 2020 );FOIG_12215和FOIG_09392的同源基因参与调控尖孢镰孢菌甜瓜专化型精氨酸的合成(Namikiet al. 2001 );FOIG_12832和FOIG_03926的同源基因参与调控白念珠菌甘氨酸的合成(McNeilet al. 2000 );FOIG_05323的同源基因参与调控烟曲霉支链氨基酸的合成(Oliveret al. 2012 );FOIG_02315的同源基因参与调控白念珠菌天冬氨酸的合成(Dahalet al. 2020 );FOIG_01066、FOIG_01154、FOIG_10878、FOIG_02315、FOIG_07232、FOIG_07170、FOIG_03322、FOIG_05025、FOIG_10638、FOIG_07565、FOIG_0314711个基因的同源基因参与调控真菌的生长发育或产孢(Puyeskyet al. 1997 ;Baidyaroyet al. 2001 ;Rodakiet al. 2006 ;Dinget al. 2010 ;Kuret al. 2010 ;Gerkeet al. 2012 ;Fuet al. 2013 ;Duet al. 2014 ;Fernandezet al. 2014 ;Dahalet al. 2020 ;Schaeferet al. 2020 );FOIG_00393、FOIG_01066、FOIG_12215、FOIG_05468、FOIG_05352、FOIG_05323、FOIG_06701、FOIG_06470、FOIG_09550、FOIG_05525、FOIG_09392、FOIG_07410、FOIG_07232、FOIG_05897、FOIG_03295、FOIG_05025、FOIG_15020、FOIG_07593、FOIG_0756519个基因的同源基因参与调控真菌的致病性或毒素产生(Chenget al. 1999 ;Baidyaroyet al. 2001 ;Namikiet al. 2001 ;Liebmannet al. 2004 ;Seonget al. 2005 ;Dinget al. 2010 ;Kingsbury & McCusker 2010;Oliveret al. 2012 ;Fuet al. 2013 ;Yaoet al. 2013 ;Duet al. 2014 ;Liet al. 2018 ;Guoet al. 2019 ;Queet al. 2020 ;Schaeferet al. 2020 ;Weiet al. 2020 )(表1).由此说明,氨基酸合成通路不仅与尖孢镰孢菌古巴专化型的氨基酸合成和厚垣孢子形成相关,其有可能参与调控该病菌的致病性. ...
Fusarial wilt of Cavendish bananas in Taiwan
1
1986
... 香蕉枯萎病是由尖孢镰孢菌古巴专化型 Fusarium oxysporum f. sp. cubense侵染引起的一种土传真菌病害,现已成为热带和亚热带地区香蕉产业的重大威胁(Ploetz 2006).根据对不同香蕉品种的致病力差异分析,该病菌被分为3个生理小种(1、2和4号生理小种),其中1号生理小种侵染大密哈(Gros Michel,AAA)和基因型为AAB的香蕉品种;2号生理小种侵染棱香蕉(Bluggoe,AAB);4号生理小种几乎侵染所有的香蕉品种,给种植户造成了严重的经济损失(Su et al. 1986).该病菌产生3种类型的孢子,分别为大型分生孢子、小型分生孢子和厚垣孢子;其中大型分生孢子和小型分生孢子是次级侵染源,多形成于香蕉寄主根部和假茎的表面;厚垣孢子是一种厚壁休眠孢子,是初级侵染源,其可在土壤中存活多年甚至10年以上.当厚垣孢子受到香蕉根部分泌物的信号刺激后,会萌发形成菌丝,侵染香蕉寄主,导致发生香蕉枯萎病(Katan et al. 1997;Ohara & Tsuge 2004;丁兆建等2019a). ...
Puccinia striiformis f. sp. tritici microRNA-like RNA 1 (Pst-milR1), an important pathogenicity factor of Pst, impairs wheat resistance to Pst by suppressing the wheat pathogenesis-related 2 gene
1
2017
... 尖孢镰孢菌古巴专化型的致病因子主要包括细胞壁降解酶、信号转导通路、毒素、sRNAs和致病相关基因.在病原菌和寄主互作过程中,病原菌通过分泌一系列细胞壁降解酶降解寄主植物的细胞壁,进而将多糖等营养物质从植物细胞内释放供病原菌生长,促进病原菌的侵入和定殖;多聚半乳糖醛酸酶已被鉴定为尖孢镰孢菌古巴专化型4号生理小种的致病因子(董章勇和王振中 2012).已有报道表明,FoSlt2 MAPK信号转导通路调控尖孢镰孢菌古巴专化型的生理特性和致病性(Dinget al. 2015 ;Dinget al. 2018a ;丁兆建等 2019b;Dinget al. 2020 );cAMP-PKA信号通路G蛋白亚基FGA2和FGB1参与调控尖孢镰孢菌古巴专化型的生长发育、产孢、耐热性和致病性(Guoet al. 2016 ).镰孢菌酸(fusaric acid,FA)是镰孢菌产生的非寄主专化性毒素,对动物和人类具有中低毒性,但对植物有较高的毒性,可引起多种作物的萎蔫病和根腐病害(Brownet al. 2012 ;Niehauset al. 2014 ).国内外已报道尖孢镰孢菌古巴专化型的多个致萎相关毒素,包括FA、白僵菌素和伏马菌素B1(许文耀等 2004;陈石等 2011;李春雨等 2011;Ding et al. 2018b ;Portal et al. 2018 ).关于植物病原真菌致病相关sRNAs报道较多,灰霉菌Botrytis cinerea、大丽轮枝菌Verticillium dahlia、小麦柄锈菌Puccinia striiformis f. sp. tritici和卵菌Oomycetes产生的sRNAs效应子可跨界传输到寄主细胞中,通过沉默寄主相关免疫基因,抑制寄主植物的免疫反应(Weiberget al. 2013 ;Wanget al. 2016 ;Wanget al. 2017 );关于尖孢镰孢菌古巴专化型sRNAs的研究处于起步阶段,李敏慧等(2019)发现用该病菌接种香蕉苗根部24h后,病原菌编码Dicer 和Argonaute的基因表达显著升高,sRNA测序结果也表明,大量milRNA显著高表达.也有多个相关致病基因得到鉴定,如活性氧迸发转录因子Foatf1(Qiet al. 2013 )、编码α-1,6-甘露糖转移酶的基因FoOCH1(Liet al. 2014 )、微管蛋白β1和β2(刘远征等 2018a,2018b)和Ⅴ型肌球蛋白Myosin5的协作蛋白smy1(何壮等 2019).尽管该菌的致病机理研究已取得了显著进展,但其致病机制仍不清晰,尤其对作为初侵染源的厚垣孢子的研究也尚未引起足够的重视. ...
Bidirectional cross-kingdom RNAi and fungal uptake of external RNAs confer plant protection
1
2016
... 尖孢镰孢菌古巴专化型的致病因子主要包括细胞壁降解酶、信号转导通路、毒素、sRNAs和致病相关基因.在病原菌和寄主互作过程中,病原菌通过分泌一系列细胞壁降解酶降解寄主植物的细胞壁,进而将多糖等营养物质从植物细胞内释放供病原菌生长,促进病原菌的侵入和定殖;多聚半乳糖醛酸酶已被鉴定为尖孢镰孢菌古巴专化型4号生理小种的致病因子(董章勇和王振中 2012).已有报道表明,FoSlt2 MAPK信号转导通路调控尖孢镰孢菌古巴专化型的生理特性和致病性(Dinget al. 2015 ;Dinget al. 2018a ;丁兆建等 2019b;Dinget al. 2020 );cAMP-PKA信号通路G蛋白亚基FGA2和FGB1参与调控尖孢镰孢菌古巴专化型的生长发育、产孢、耐热性和致病性(Guoet al. 2016 ).镰孢菌酸(fusaric acid,FA)是镰孢菌产生的非寄主专化性毒素,对动物和人类具有中低毒性,但对植物有较高的毒性,可引起多种作物的萎蔫病和根腐病害(Brownet al. 2012 ;Niehauset al. 2014 ).国内外已报道尖孢镰孢菌古巴专化型的多个致萎相关毒素,包括FA、白僵菌素和伏马菌素B1(许文耀等 2004;陈石等 2011;李春雨等 2011;Ding et al. 2018b ;Portal et al. 2018 ).关于植物病原真菌致病相关sRNAs报道较多,灰霉菌Botrytis cinerea、大丽轮枝菌Verticillium dahlia、小麦柄锈菌Puccinia striiformis f. sp. tritici和卵菌Oomycetes产生的sRNAs效应子可跨界传输到寄主细胞中,通过沉默寄主相关免疫基因,抑制寄主植物的免疫反应(Weiberget al. 2013 ;Wanget al. 2016 ;Wanget al. 2017 );关于尖孢镰孢菌古巴专化型sRNAs的研究处于起步阶段,李敏慧等(2019)发现用该病菌接种香蕉苗根部24h后,病原菌编码Dicer 和Argonaute的基因表达显著升高,sRNA测序结果也表明,大量milRNA显著高表达.也有多个相关致病基因得到鉴定,如活性氧迸发转录因子Foatf1(Qiet al. 2013 )、编码α-1,6-甘露糖转移酶的基因FoOCH1(Liet al. 2014 )、微管蛋白β1和β2(刘远征等 2018a,2018b)和Ⅴ型肌球蛋白Myosin5的协作蛋白smy1(何壮等 2019).尽管该菌的致病机理研究已取得了显著进展,但其致病机制仍不清晰,尤其对作为初侵染源的厚垣孢子的研究也尚未引起足够的重视. ...
Host-induced gene silencing of the acetolactate synthases VdILV2 and VdILV6 confers resistance to Verticillium wilt in cotton (Gossypium hirsutum L.)
3
2020
... 氨基酸是重要的有机化合物,其在蛋白质合成、细胞生长、发育和能量产生等多个生物学过程中起着重要作用.已有研究证明氨基酸代谢与真菌的产孢、生长和致病性相关.在人类致病菌中,赖氨酸合成基因lysF调控烟曲霉Aspergillus fumigatus的致病性(Liebmannet al. 2004 );3个精氨酸合成相关基因Car1、Agt1和Gbu1调控白念珠菌Candida albicans的生长和致病性(Schaeferet al. 2020 );天冬氨酸合成基因asd的缺失抑制白念珠菌的生长(Dahalet al. 2020 ).L-精氨酸是生防菌盾壳霉Coniothyrium minitans产孢所必需的,其衍生物一氧化氮可能介导其产孢功能(Gonget al. 2007 ).在植物病原菌中,尖孢镰孢菌甜瓜专化型F.oxysporum f. sp. melonis精氨酸合成基因ARG1缺失突变体(Namikiet al. 2001 )和灰霉菌精氨酸合成基因bcass1缺失突变体(Patelet al. 2010 )的致病性均减弱;甲硫氨酸合成基因FgMETB调控禾谷镰孢菌的孢子萌发、菌丝生长、DON毒素产生和致病性(Fuet al. 2013 );谷氨酸合成基因P5Cdh调控栗疫病菌Cryphonectria parasitica的致病性和产孢(Yaoet al. 2013 );甲硫氨酸合成基因MET13(Yanet al. 2013 )、赖氨酸合成基因MoLys2(Chenet al. 2014 )、苏氨酸合成基因MoIlv1(Duet al. 2014 )、精氨酸合成基因(MoARG1、MoARG5、MoARG6 和 MoARG7)(Zhanget al. 2015 )、亮氨酸合成基因(MoLEU1、MoLEU2、MoLEU4、LEU1、LEU2A和LEU4)(Queet al. 2020 ;Weiet al. 2020 )与稻瘟菌的生理特性和致病性密切相关.迄今,尚未有氨基酸与尖孢镰孢菌古巴专化型产孢、生长发育和致病性的相关报道. ...
... 通过对氨基酸合成通路93个差异表达基因的In silico分析,发现其中部分差异表达基因在其他真菌已得到鉴定,如FOIG_05525、FOIG_05468、FOIG_09550和FOIG_075934个基因的同源基因参与调控真菌亮氨酸的合成(Liebmannet al. 2004 ;Queet al. 2020 );FOIG_12215和FOIG_09392的同源基因参与调控尖孢镰孢菌甜瓜专化型精氨酸的合成(Namikiet al. 2001 );FOIG_12832和FOIG_03926的同源基因参与调控白念珠菌甘氨酸的合成(McNeilet al. 2000 );FOIG_05323的同源基因参与调控烟曲霉支链氨基酸的合成(Oliveret al. 2012 );FOIG_02315的同源基因参与调控白念珠菌天冬氨酸的合成(Dahalet al. 2020 );FOIG_01066、FOIG_01154、FOIG_10878、FOIG_02315、FOIG_07232、FOIG_07170、FOIG_03322、FOIG_05025、FOIG_10638、FOIG_07565、FOIG_0314711个基因的同源基因参与调控真菌的生长发育或产孢(Puyeskyet al. 1997 ;Baidyaroyet al. 2001 ;Rodakiet al. 2006 ;Dinget al. 2010 ;Kuret al. 2010 ;Gerkeet al. 2012 ;Fuet al. 2013 ;Duet al. 2014 ;Fernandezet al. 2014 ;Dahalet al. 2020 ;Schaeferet al. 2020 );FOIG_00393、FOIG_01066、FOIG_12215、FOIG_05468、FOIG_05352、FOIG_05323、FOIG_06701、FOIG_06470、FOIG_09550、FOIG_05525、FOIG_09392、FOIG_07410、FOIG_07232、FOIG_05897、FOIG_03295、FOIG_05025、FOIG_15020、FOIG_07593、FOIG_0756519个基因的同源基因参与调控真菌的致病性或毒素产生(Chenget al. 1999 ;Baidyaroyet al. 2001 ;Namikiet al. 2001 ;Liebmannet al. 2004 ;Seonget al. 2005 ;Dinget al. 2010 ;Kingsbury & McCusker 2010;Oliveret al. 2012 ;Fuet al. 2013 ;Yaoet al. 2013 ;Duet al. 2014 ;Liet al. 2018 ;Guoet al. 2019 ;Queet al. 2020 ;Schaeferet al. 2020 ;Weiet al. 2020 )(表1).由此说明,氨基酸合成通路不仅与尖孢镰孢菌古巴专化型的氨基酸合成和厚垣孢子形成相关,其有可能参与调控该病菌的致病性. ...
... 氨基酸合成通路通过调控氨基酸的合成,影响尖孢镰孢菌古巴专化型厚垣孢子的形成.前人的研究表明,氨基酸合成基因的敲除能导致氨基酸的合成缺失,同时影响真菌生长或产孢,如白念珠菌精氨酸合成基因 Car1、Agt1和Gbu1(Schaeferet al. 2020 )及天冬氨酸合成基因asd(Dahalet al. 2020 ),禾谷镰孢菌甲硫氨酸合成基因FgMETB(Fuet al. 2013 )、栗疫病菌谷氨酸合成基因 P5Cdh(Yaoet al. 2013 )、稻瘟菌甲硫氨酸合成基因MET13(Yanet al. 2013 )、赖氨酸合成基因MoLys2(Chenet al. 2014 )、苏氨酸合成基因MoIlv1(Duet al. 2014 )、精氨酸合成基因(MoARG1、MoARG5、MoARG6和MoARG7)(Zhanget al. 2015 )、亮氨酸合成基因(MoLEU1、MoLEU2、MoLEU4、LEU1、LEU2A 和LEU4)(Queet al. 2020 ;Weiet al. 2020 ).值得指出的是,这些氨基酸合成基因的同源基因的表达水平在尖孢镰孢菌古巴专化型厚垣孢子形成过程中均发生了变化.此外,本研究发现外源甘氨酸抑制尖孢镰孢菌古巴专化型厚垣孢子的诱导形成.这些数据确认了氨基酸合成基因与尖孢镰孢菌古巴专化型厚垣孢子形成存在联系,氨基酸合成通路可通过调控氨基酸合成基因的转录,进而影响尖孢镰孢菌古巴专化型厚垣孢子的形成. ...
Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways
1
2013
... 尖孢镰孢菌古巴专化型的致病因子主要包括细胞壁降解酶、信号转导通路、毒素、sRNAs和致病相关基因.在病原菌和寄主互作过程中,病原菌通过分泌一系列细胞壁降解酶降解寄主植物的细胞壁,进而将多糖等营养物质从植物细胞内释放供病原菌生长,促进病原菌的侵入和定殖;多聚半乳糖醛酸酶已被鉴定为尖孢镰孢菌古巴专化型4号生理小种的致病因子(董章勇和王振中 2012).已有报道表明,FoSlt2 MAPK信号转导通路调控尖孢镰孢菌古巴专化型的生理特性和致病性(Dinget al. 2015 ;Dinget al. 2018a ;丁兆建等 2019b;Dinget al. 2020 );cAMP-PKA信号通路G蛋白亚基FGA2和FGB1参与调控尖孢镰孢菌古巴专化型的生长发育、产孢、耐热性和致病性(Guoet al. 2016 ).镰孢菌酸(fusaric acid,FA)是镰孢菌产生的非寄主专化性毒素,对动物和人类具有中低毒性,但对植物有较高的毒性,可引起多种作物的萎蔫病和根腐病害(Brownet al. 2012 ;Niehauset al. 2014 ).国内外已报道尖孢镰孢菌古巴专化型的多个致萎相关毒素,包括FA、白僵菌素和伏马菌素B1(许文耀等 2004;陈石等 2011;李春雨等 2011;Ding et al. 2018b ;Portal et al. 2018 ).关于植物病原真菌致病相关sRNAs报道较多,灰霉菌Botrytis cinerea、大丽轮枝菌Verticillium dahlia、小麦柄锈菌Puccinia striiformis f. sp. tritici和卵菌Oomycetes产生的sRNAs效应子可跨界传输到寄主细胞中,通过沉默寄主相关免疫基因,抑制寄主植物的免疫反应(Weiberget al. 2013 ;Wanget al. 2016 ;Wanget al. 2017 );关于尖孢镰孢菌古巴专化型sRNAs的研究处于起步阶段,李敏慧等(2019)发现用该病菌接种香蕉苗根部24h后,病原菌编码Dicer 和Argonaute的基因表达显著升高,sRNA测序结果也表明,大量milRNA显著高表达.也有多个相关致病基因得到鉴定,如活性氧迸发转录因子Foatf1(Qiet al. 2013 )、编码α-1,6-甘露糖转移酶的基因FoOCH1(Liet al. 2014 )、微管蛋白β1和β2(刘远征等 2018a,2018b)和Ⅴ型肌球蛋白Myosin5的协作蛋白smy1(何壮等 2019).尽管该菌的致病机理研究已取得了显著进展,但其致病机制仍不清晰,尤其对作为初侵染源的厚垣孢子的研究也尚未引起足够的重视. ...
The toxicity of the crude toxin of Fusarium oxysporum f. sp. cubense and its model
0
2004
The MET13 methylenetetrahydrofolate reductase gene is essential for infection-related morphogenesis in the rice blast fungus Magnaporthe oryzae
2
2013
... 氨基酸是重要的有机化合物,其在蛋白质合成、细胞生长、发育和能量产生等多个生物学过程中起着重要作用.已有研究证明氨基酸代谢与真菌的产孢、生长和致病性相关.在人类致病菌中,赖氨酸合成基因lysF调控烟曲霉Aspergillus fumigatus的致病性(Liebmannet al. 2004 );3个精氨酸合成相关基因Car1、Agt1和Gbu1调控白念珠菌Candida albicans的生长和致病性(Schaeferet al. 2020 );天冬氨酸合成基因asd的缺失抑制白念珠菌的生长(Dahalet al. 2020 ).L-精氨酸是生防菌盾壳霉Coniothyrium minitans产孢所必需的,其衍生物一氧化氮可能介导其产孢功能(Gonget al. 2007 ).在植物病原菌中,尖孢镰孢菌甜瓜专化型F.oxysporum f. sp. melonis精氨酸合成基因ARG1缺失突变体(Namikiet al. 2001 )和灰霉菌精氨酸合成基因bcass1缺失突变体(Patelet al. 2010 )的致病性均减弱;甲硫氨酸合成基因FgMETB调控禾谷镰孢菌的孢子萌发、菌丝生长、DON毒素产生和致病性(Fuet al. 2013 );谷氨酸合成基因P5Cdh调控栗疫病菌Cryphonectria parasitica的致病性和产孢(Yaoet al. 2013 );甲硫氨酸合成基因MET13(Yanet al. 2013 )、赖氨酸合成基因MoLys2(Chenet al. 2014 )、苏氨酸合成基因MoIlv1(Duet al. 2014 )、精氨酸合成基因(MoARG1、MoARG5、MoARG6 和 MoARG7)(Zhanget al. 2015 )、亮氨酸合成基因(MoLEU1、MoLEU2、MoLEU4、LEU1、LEU2A和LEU4)(Queet al. 2020 ;Weiet al. 2020 )与稻瘟菌的生理特性和致病性密切相关.迄今,尚未有氨基酸与尖孢镰孢菌古巴专化型产孢、生长发育和致病性的相关报道. ...
... 氨基酸合成通路通过调控氨基酸的合成,影响尖孢镰孢菌古巴专化型厚垣孢子的形成.前人的研究表明,氨基酸合成基因的敲除能导致氨基酸的合成缺失,同时影响真菌生长或产孢,如白念珠菌精氨酸合成基因 Car1、Agt1和Gbu1(Schaeferet al. 2020 )及天冬氨酸合成基因asd(Dahalet al. 2020 ),禾谷镰孢菌甲硫氨酸合成基因FgMETB(Fuet al. 2013 )、栗疫病菌谷氨酸合成基因 P5Cdh(Yaoet al. 2013 )、稻瘟菌甲硫氨酸合成基因MET13(Yanet al. 2013 )、赖氨酸合成基因MoLys2(Chenet al. 2014 )、苏氨酸合成基因MoIlv1(Duet al. 2014 )、精氨酸合成基因(MoARG1、MoARG5、MoARG6和MoARG7)(Zhanget al. 2015 )、亮氨酸合成基因(MoLEU1、MoLEU2、MoLEU4、LEU1、LEU2A 和LEU4)(Queet al. 2020 ;Weiet al. 2020 ).值得指出的是,这些氨基酸合成基因的同源基因的表达水平在尖孢镰孢菌古巴专化型厚垣孢子形成过程中均发生了变化.此外,本研究发现外源甘氨酸抑制尖孢镰孢菌古巴专化型厚垣孢子的诱导形成.这些数据确认了氨基酸合成基因与尖孢镰孢菌古巴专化型厚垣孢子形成存在联系,氨基酸合成通路可通过调控氨基酸合成基因的转录,进而影响尖孢镰孢菌古巴专化型厚垣孢子的形成. ...
Δ1-pyrroline-5-carboxylate/glutamate biogenesis is required for fungal virulence and sporulation
3
2013
... 氨基酸是重要的有机化合物,其在蛋白质合成、细胞生长、发育和能量产生等多个生物学过程中起着重要作用.已有研究证明氨基酸代谢与真菌的产孢、生长和致病性相关.在人类致病菌中,赖氨酸合成基因lysF调控烟曲霉Aspergillus fumigatus的致病性(Liebmannet al. 2004 );3个精氨酸合成相关基因Car1、Agt1和Gbu1调控白念珠菌Candida albicans的生长和致病性(Schaeferet al. 2020 );天冬氨酸合成基因asd的缺失抑制白念珠菌的生长(Dahalet al. 2020 ).L-精氨酸是生防菌盾壳霉Coniothyrium minitans产孢所必需的,其衍生物一氧化氮可能介导其产孢功能(Gonget al. 2007 ).在植物病原菌中,尖孢镰孢菌甜瓜专化型F.oxysporum f. sp. melonis精氨酸合成基因ARG1缺失突变体(Namikiet al. 2001 )和灰霉菌精氨酸合成基因bcass1缺失突变体(Patelet al. 2010 )的致病性均减弱;甲硫氨酸合成基因FgMETB调控禾谷镰孢菌的孢子萌发、菌丝生长、DON毒素产生和致病性(Fuet al. 2013 );谷氨酸合成基因P5Cdh调控栗疫病菌Cryphonectria parasitica的致病性和产孢(Yaoet al. 2013 );甲硫氨酸合成基因MET13(Yanet al. 2013 )、赖氨酸合成基因MoLys2(Chenet al. 2014 )、苏氨酸合成基因MoIlv1(Duet al. 2014 )、精氨酸合成基因(MoARG1、MoARG5、MoARG6 和 MoARG7)(Zhanget al. 2015 )、亮氨酸合成基因(MoLEU1、MoLEU2、MoLEU4、LEU1、LEU2A和LEU4)(Queet al. 2020 ;Weiet al. 2020 )与稻瘟菌的生理特性和致病性密切相关.迄今,尚未有氨基酸与尖孢镰孢菌古巴专化型产孢、生长发育和致病性的相关报道. ...
... 通过对氨基酸合成通路93个差异表达基因的In silico分析,发现其中部分差异表达基因在其他真菌已得到鉴定,如FOIG_05525、FOIG_05468、FOIG_09550和FOIG_075934个基因的同源基因参与调控真菌亮氨酸的合成(Liebmannet al. 2004 ;Queet al. 2020 );FOIG_12215和FOIG_09392的同源基因参与调控尖孢镰孢菌甜瓜专化型精氨酸的合成(Namikiet al. 2001 );FOIG_12832和FOIG_03926的同源基因参与调控白念珠菌甘氨酸的合成(McNeilet al. 2000 );FOIG_05323的同源基因参与调控烟曲霉支链氨基酸的合成(Oliveret al. 2012 );FOIG_02315的同源基因参与调控白念珠菌天冬氨酸的合成(Dahalet al. 2020 );FOIG_01066、FOIG_01154、FOIG_10878、FOIG_02315、FOIG_07232、FOIG_07170、FOIG_03322、FOIG_05025、FOIG_10638、FOIG_07565、FOIG_0314711个基因的同源基因参与调控真菌的生长发育或产孢(Puyeskyet al. 1997 ;Baidyaroyet al. 2001 ;Rodakiet al. 2006 ;Dinget al. 2010 ;Kuret al. 2010 ;Gerkeet al. 2012 ;Fuet al. 2013 ;Duet al. 2014 ;Fernandezet al. 2014 ;Dahalet al. 2020 ;Schaeferet al. 2020 );FOIG_00393、FOIG_01066、FOIG_12215、FOIG_05468、FOIG_05352、FOIG_05323、FOIG_06701、FOIG_06470、FOIG_09550、FOIG_05525、FOIG_09392、FOIG_07410、FOIG_07232、FOIG_05897、FOIG_03295、FOIG_05025、FOIG_15020、FOIG_07593、FOIG_0756519个基因的同源基因参与调控真菌的致病性或毒素产生(Chenget al. 1999 ;Baidyaroyet al. 2001 ;Namikiet al. 2001 ;Liebmannet al. 2004 ;Seonget al. 2005 ;Dinget al. 2010 ;Kingsbury & McCusker 2010;Oliveret al. 2012 ;Fuet al. 2013 ;Yaoet al. 2013 ;Duet al. 2014 ;Liet al. 2018 ;Guoet al. 2019 ;Queet al. 2020 ;Schaeferet al. 2020 ;Weiet al. 2020 )(表1).由此说明,氨基酸合成通路不仅与尖孢镰孢菌古巴专化型的氨基酸合成和厚垣孢子形成相关,其有可能参与调控该病菌的致病性. ...
... 氨基酸合成通路通过调控氨基酸的合成,影响尖孢镰孢菌古巴专化型厚垣孢子的形成.前人的研究表明,氨基酸合成基因的敲除能导致氨基酸的合成缺失,同时影响真菌生长或产孢,如白念珠菌精氨酸合成基因 Car1、Agt1和Gbu1(Schaeferet al. 2020 )及天冬氨酸合成基因asd(Dahalet al. 2020 ),禾谷镰孢菌甲硫氨酸合成基因FgMETB(Fuet al. 2013 )、栗疫病菌谷氨酸合成基因 P5Cdh(Yaoet al. 2013 )、稻瘟菌甲硫氨酸合成基因MET13(Yanet al. 2013 )、赖氨酸合成基因MoLys2(Chenet al. 2014 )、苏氨酸合成基因MoIlv1(Duet al. 2014 )、精氨酸合成基因(MoARG1、MoARG5、MoARG6和MoARG7)(Zhanget al. 2015 )、亮氨酸合成基因(MoLEU1、MoLEU2、MoLEU4、LEU1、LEU2A 和LEU4)(Queet al. 2020 ;Weiet al. 2020 ).值得指出的是,这些氨基酸合成基因的同源基因的表达水平在尖孢镰孢菌古巴专化型厚垣孢子形成过程中均发生了变化.此外,本研究发现外源甘氨酸抑制尖孢镰孢菌古巴专化型厚垣孢子的诱导形成.这些数据确认了氨基酸合成基因与尖孢镰孢菌古巴专化型厚垣孢子形成存在联系,氨基酸合成通路可通过调控氨基酸合成基因的转录,进而影响尖孢镰孢菌古巴专化型厚垣孢子的形成. ...
MoARG1, MoARG5,6 and MoARG7 involved in arginine biosynthesis are essential for growth, conidiogenesis, sexual reproduction, and pathogenicity in Magnaporthe oryzae
2
2015
... 氨基酸是重要的有机化合物,其在蛋白质合成、细胞生长、发育和能量产生等多个生物学过程中起着重要作用.已有研究证明氨基酸代谢与真菌的产孢、生长和致病性相关.在人类致病菌中,赖氨酸合成基因lysF调控烟曲霉Aspergillus fumigatus的致病性(Liebmannet al. 2004 );3个精氨酸合成相关基因Car1、Agt1和Gbu1调控白念珠菌Candida albicans的生长和致病性(Schaeferet al. 2020 );天冬氨酸合成基因asd的缺失抑制白念珠菌的生长(Dahalet al. 2020 ).L-精氨酸是生防菌盾壳霉Coniothyrium minitans产孢所必需的,其衍生物一氧化氮可能介导其产孢功能(Gonget al. 2007 ).在植物病原菌中,尖孢镰孢菌甜瓜专化型F.oxysporum f. sp. melonis精氨酸合成基因ARG1缺失突变体(Namikiet al. 2001 )和灰霉菌精氨酸合成基因bcass1缺失突变体(Patelet al. 2010 )的致病性均减弱;甲硫氨酸合成基因FgMETB调控禾谷镰孢菌的孢子萌发、菌丝生长、DON毒素产生和致病性(Fuet al. 2013 );谷氨酸合成基因P5Cdh调控栗疫病菌Cryphonectria parasitica的致病性和产孢(Yaoet al. 2013 );甲硫氨酸合成基因MET13(Yanet al. 2013 )、赖氨酸合成基因MoLys2(Chenet al. 2014 )、苏氨酸合成基因MoIlv1(Duet al. 2014 )、精氨酸合成基因(MoARG1、MoARG5、MoARG6 和 MoARG7)(Zhanget al. 2015 )、亮氨酸合成基因(MoLEU1、MoLEU2、MoLEU4、LEU1、LEU2A和LEU4)(Queet al. 2020 ;Weiet al. 2020 )与稻瘟菌的生理特性和致病性密切相关.迄今,尚未有氨基酸与尖孢镰孢菌古巴专化型产孢、生长发育和致病性的相关报道. ...
... 氨基酸合成通路通过调控氨基酸的合成,影响尖孢镰孢菌古巴专化型厚垣孢子的形成.前人的研究表明,氨基酸合成基因的敲除能导致氨基酸的合成缺失,同时影响真菌生长或产孢,如白念珠菌精氨酸合成基因 Car1、Agt1和Gbu1(Schaeferet al. 2020 )及天冬氨酸合成基因asd(Dahalet al. 2020 ),禾谷镰孢菌甲硫氨酸合成基因FgMETB(Fuet al. 2013 )、栗疫病菌谷氨酸合成基因 P5Cdh(Yaoet al. 2013 )、稻瘟菌甲硫氨酸合成基因MET13(Yanet al. 2013 )、赖氨酸合成基因MoLys2(Chenet al. 2014 )、苏氨酸合成基因MoIlv1(Duet al. 2014 )、精氨酸合成基因(MoARG1、MoARG5、MoARG6和MoARG7)(Zhanget al. 2015 )、亮氨酸合成基因(MoLEU1、MoLEU2、MoLEU4、LEU1、LEU2A 和LEU4)(Queet al. 2020 ;Weiet al. 2020 ).值得指出的是,这些氨基酸合成基因的同源基因的表达水平在尖孢镰孢菌古巴专化型厚垣孢子形成过程中均发生了变化.此外,本研究发现外源甘氨酸抑制尖孢镰孢菌古巴专化型厚垣孢子的诱导形成.这些数据确认了氨基酸合成基因与尖孢镰孢菌古巴专化型厚垣孢子形成存在联系,氨基酸合成通路可通过调控氨基酸合成基因的转录,进而影响尖孢镰孢菌古巴专化型厚垣孢子的形成. ...
尖镰孢菌致病机理研究进展
1
2011
... 尖孢镰孢菌古巴专化型的致病因子主要包括细胞壁降解酶、信号转导通路、毒素、sRNAs和致病相关基因.在病原菌和寄主互作过程中,病原菌通过分泌一系列细胞壁降解酶降解寄主植物的细胞壁,进而将多糖等营养物质从植物细胞内释放供病原菌生长,促进病原菌的侵入和定殖;多聚半乳糖醛酸酶已被鉴定为尖孢镰孢菌古巴专化型4号生理小种的致病因子(董章勇和王振中 2012).已有报道表明,FoSlt2 MAPK信号转导通路调控尖孢镰孢菌古巴专化型的生理特性和致病性(Dinget al. 2015 ;Dinget al. 2018a ;丁兆建等 2019b;Dinget al. 2020 );cAMP-PKA信号通路G蛋白亚基FGA2和FGB1参与调控尖孢镰孢菌古巴专化型的生长发育、产孢、耐热性和致病性(Guoet al. 2016 ).镰孢菌酸(fusaric acid,FA)是镰孢菌产生的非寄主专化性毒素,对动物和人类具有中低毒性,但对植物有较高的毒性,可引起多种作物的萎蔫病和根腐病害(Brownet al. 2012 ;Niehauset al. 2014 ).国内外已报道尖孢镰孢菌古巴专化型的多个致萎相关毒素,包括FA、白僵菌素和伏马菌素B1(许文耀等 2004;陈石等 2011;李春雨等 2011;Ding et al. 2018b ;Portal et al. 2018 ).关于植物病原真菌致病相关sRNAs报道较多,灰霉菌Botrytis cinerea、大丽轮枝菌Verticillium dahlia、小麦柄锈菌Puccinia striiformis f. sp. tritici和卵菌Oomycetes产生的sRNAs效应子可跨界传输到寄主细胞中,通过沉默寄主相关免疫基因,抑制寄主植物的免疫反应(Weiberget al. 2013 ;Wanget al. 2016 ;Wanget al. 2017 );关于尖孢镰孢菌古巴专化型sRNAs的研究处于起步阶段,李敏慧等(2019)发现用该病菌接种香蕉苗根部24h后,病原菌编码Dicer 和Argonaute的基因表达显著升高,sRNA测序结果也表明,大量milRNA显著高表达.也有多个相关致病基因得到鉴定,如活性氧迸发转录因子Foatf1(Qiet al. 2013 )、编码α-1,6-甘露糖转移酶的基因FoOCH1(Liet al. 2014 )、微管蛋白β1和β2(刘远征等 2018a,2018b)和Ⅴ型肌球蛋白Myosin5的协作蛋白smy1(何壮等 2019).尽管该菌的致病机理研究已取得了显著进展,但其致病机制仍不清晰,尤其对作为初侵染源的厚垣孢子的研究也尚未引起足够的重视. ...
转录因子FoSwi6调控香蕉枯萎病菌的生理特性和致病性
1
2018a
... 本研究采用的菌株尖孢镰孢菌古巴专化型4号生理小种(Foc4)(丁兆建等 2018a),为本实验室保存菌株.采用马铃薯葡萄糖琼脂培养基(PDA)、察氏培养基(Czapek dox medium)进行病菌培养,培养温度为28℃(丁兆建等 2019a). ...
氨基糖代谢通路影响尖孢镰刀菌古巴专化型厚垣孢子的形成
6
2019a
... 香蕉枯萎病是由尖孢镰孢菌古巴专化型 Fusarium oxysporum f. sp. cubense侵染引起的一种土传真菌病害,现已成为热带和亚热带地区香蕉产业的重大威胁(Ploetz 2006).根据对不同香蕉品种的致病力差异分析,该病菌被分为3个生理小种(1、2和4号生理小种),其中1号生理小种侵染大密哈(Gros Michel,AAA)和基因型为AAB的香蕉品种;2号生理小种侵染棱香蕉(Bluggoe,AAB);4号生理小种几乎侵染所有的香蕉品种,给种植户造成了严重的经济损失(Su et al. 1986).该病菌产生3种类型的孢子,分别为大型分生孢子、小型分生孢子和厚垣孢子;其中大型分生孢子和小型分生孢子是次级侵染源,多形成于香蕉寄主根部和假茎的表面;厚垣孢子是一种厚壁休眠孢子,是初级侵染源,其可在土壤中存活多年甚至10年以上.当厚垣孢子受到香蕉根部分泌物的信号刺激后,会萌发形成菌丝,侵染香蕉寄主,导致发生香蕉枯萎病(Katan et al. 1997;Ohara & Tsuge 2004;丁兆建等2019a). ...
... 本研究采用的菌株尖孢镰孢菌古巴专化型4号生理小种(Foc4)(丁兆建等 2018a),为本实验室保存菌株.采用马铃薯葡萄糖琼脂培养基(PDA)、察氏培养基(Czapek dox medium)进行病菌培养,培养温度为28℃(丁兆建等 2019a). ...
... 为了检测氨基酸是否影响尖孢镰孢菌古巴专化型厚垣孢子的形成,本研究开展了甘氨酸(glycine)添加试验,即在该病菌厚垣孢子的诱导形成体系中(丁兆建等 2019a)添加甘氨酸,使其终浓度达到10g/L或者20g/L,96h后观察厚垣孢子的形成情况,以不添加甘氨酸处理作为阳性对照. ...
... 根据丁兆建等(2019a)建立的方法进行厚垣孢子定量分级,稍加修改,具体分为4级:利用光学显微镜观察3张玻片(每张玻片不少于5个视野)共计不少于15个视野,视野中无厚垣孢子产生为0级;厚垣孢子覆盖的视野面积小于1/5为厚垣孢子少量产生,定为1级;厚垣孢子覆盖的视野面积大于或等于1/5但小于1/2为厚垣孢子中等产生,定为2级;厚垣孢子覆盖的视野面积大于或等于1/2为厚垣孢子大量产生,定为3级. ...
... 利用OmicShare热图工具对厚垣孢子形成发育过程中,富集在氨基酸合成通路的差异表达基因的FPKM(fragments per kilobase per million)值(丁兆建等 2019a),进行基因表达量和聚类分析. ...
... 氨基酸如何影响真菌产孢、生长或者抑制尖孢镰孢菌古巴专化型厚垣孢子形成呢?真菌为适应不同或者变化的环境,会尽可能广泛地选择营养来源.有些真菌种类利用氨基酸的降解产物作为唯一氮源,有的则利用其作为唯一碳源,有的则两者兼有之(Rzadet al. 2018 ),如白念珠菌、新型隐球菌Cryptococcus neoformans和烟曲霉利用赖氨酸作为唯一氮源,酿酒酵母则利用赖氨酸作为唯一的碳源而非唯一氮源(Kinzelet al. 1983 ).已有报道表明,碳源的浓度与真菌厚垣孢子的形成相关,高浓度的葡萄糖抑制白色念珠菌和镰孢菌厚垣孢子的形成,缺乏或者低浓度的葡萄糖可促使镰孢菌厚垣孢子的形成(Qureshi & Page 1970;Griffin 1976;Mandal & Chaudhuri 2013;Bottcheret al. 2016 ).前期的研究发现,作为碳源的N-乙酰葡糖胺浓度达到20g/L时,尖孢镰孢菌古巴专化型的菌丝不产生厚垣孢子;当N-乙酰葡糖胺缺乏时,其菌丝可产生大量厚垣孢子(丁兆建等 2019a).由此推测,外源甘氨酸的添加导致碳源的浓度升高,从而抑制尖孢镰孢菌古巴专化型厚垣孢子的诱导形成. ...
尖孢镰刀菌古巴专化型MAPK FoSlt2敲除突变体的转录组分析
1
2019b
... 尖孢镰孢菌古巴专化型的致病因子主要包括细胞壁降解酶、信号转导通路、毒素、sRNAs和致病相关基因.在病原菌和寄主互作过程中,病原菌通过分泌一系列细胞壁降解酶降解寄主植物的细胞壁,进而将多糖等营养物质从植物细胞内释放供病原菌生长,促进病原菌的侵入和定殖;多聚半乳糖醛酸酶已被鉴定为尖孢镰孢菌古巴专化型4号生理小种的致病因子(董章勇和王振中 2012).已有报道表明,FoSlt2 MAPK信号转导通路调控尖孢镰孢菌古巴专化型的生理特性和致病性(Dinget al. 2015 ;Dinget al. 2018a ;丁兆建等 2019b;Dinget al. 2020 );cAMP-PKA信号通路G蛋白亚基FGA2和FGB1参与调控尖孢镰孢菌古巴专化型的生长发育、产孢、耐热性和致病性(Guoet al. 2016 ).镰孢菌酸(fusaric acid,FA)是镰孢菌产生的非寄主专化性毒素,对动物和人类具有中低毒性,但对植物有较高的毒性,可引起多种作物的萎蔫病和根腐病害(Brownet al. 2012 ;Niehauset al. 2014 ).国内外已报道尖孢镰孢菌古巴专化型的多个致萎相关毒素,包括FA、白僵菌素和伏马菌素B1(许文耀等 2004;陈石等 2011;李春雨等 2011;Ding et al. 2018b ;Portal et al. 2018 ).关于植物病原真菌致病相关sRNAs报道较多,灰霉菌Botrytis cinerea、大丽轮枝菌Verticillium dahlia、小麦柄锈菌Puccinia striiformis f. sp. tritici和卵菌Oomycetes产生的sRNAs效应子可跨界传输到寄主细胞中,通过沉默寄主相关免疫基因,抑制寄主植物的免疫反应(Weiberget al. 2013 ;Wanget al. 2016 ;Wanget al. 2017 );关于尖孢镰孢菌古巴专化型sRNAs的研究处于起步阶段,李敏慧等(2019)发现用该病菌接种香蕉苗根部24h后,病原菌编码Dicer 和Argonaute的基因表达显著升高,sRNA测序结果也表明,大量milRNA显著高表达.也有多个相关致病基因得到鉴定,如活性氧迸发转录因子Foatf1(Qiet al. 2013 )、编码α-1,6-甘露糖转移酶的基因FoOCH1(Liet al. 2014 )、微管蛋白β1和β2(刘远征等 2018a,2018b)和Ⅴ型肌球蛋白Myosin5的协作蛋白smy1(何壮等 2019).尽管该菌的致病机理研究已取得了显著进展,但其致病机制仍不清晰,尤其对作为初侵染源的厚垣孢子的研究也尚未引起足够的重视. ...
植物病原真菌细胞壁降解酶的研究进展
1
2012
... 尖孢镰孢菌古巴专化型的致病因子主要包括细胞壁降解酶、信号转导通路、毒素、sRNAs和致病相关基因.在病原菌和寄主互作过程中,病原菌通过分泌一系列细胞壁降解酶降解寄主植物的细胞壁,进而将多糖等营养物质从植物细胞内释放供病原菌生长,促进病原菌的侵入和定殖;多聚半乳糖醛酸酶已被鉴定为尖孢镰孢菌古巴专化型4号生理小种的致病因子(董章勇和王振中 2012).已有报道表明,FoSlt2 MAPK信号转导通路调控尖孢镰孢菌古巴专化型的生理特性和致病性(Dinget al. 2015 ;Dinget al. 2018a ;丁兆建等 2019b;Dinget al. 2020 );cAMP-PKA信号通路G蛋白亚基FGA2和FGB1参与调控尖孢镰孢菌古巴专化型的生长发育、产孢、耐热性和致病性(Guoet al. 2016 ).镰孢菌酸(fusaric acid,FA)是镰孢菌产生的非寄主专化性毒素,对动物和人类具有中低毒性,但对植物有较高的毒性,可引起多种作物的萎蔫病和根腐病害(Brownet al. 2012 ;Niehauset al. 2014 ).国内外已报道尖孢镰孢菌古巴专化型的多个致萎相关毒素,包括FA、白僵菌素和伏马菌素B1(许文耀等 2004;陈石等 2011;李春雨等 2011;Ding et al. 2018b ;Portal et al. 2018 ).关于植物病原真菌致病相关sRNAs报道较多,灰霉菌Botrytis cinerea、大丽轮枝菌Verticillium dahlia、小麦柄锈菌Puccinia striiformis f. sp. tritici和卵菌Oomycetes产生的sRNAs效应子可跨界传输到寄主细胞中,通过沉默寄主相关免疫基因,抑制寄主植物的免疫反应(Weiberget al. 2013 ;Wanget al. 2016 ;Wanget al. 2017 );关于尖孢镰孢菌古巴专化型sRNAs的研究处于起步阶段,李敏慧等(2019)发现用该病菌接种香蕉苗根部24h后,病原菌编码Dicer 和Argonaute的基因表达显著升高,sRNA测序结果也表明,大量milRNA显著高表达.也有多个相关致病基因得到鉴定,如活性氧迸发转录因子Foatf1(Qiet al. 2013 )、编码α-1,6-甘露糖转移酶的基因FoOCH1(Liet al. 2014 )、微管蛋白β1和β2(刘远征等 2018a,2018b)和Ⅴ型肌球蛋白Myosin5的协作蛋白smy1(何壮等 2019).尽管该菌的致病机理研究已取得了显著进展,但其致病机制仍不清晰,尤其对作为初侵染源的厚垣孢子的研究也尚未引起足够的重视. ...
香蕉枯萎病菌4号生理小种smy1基因的功能分析
1
2019
... 尖孢镰孢菌古巴专化型的致病因子主要包括细胞壁降解酶、信号转导通路、毒素、sRNAs和致病相关基因.在病原菌和寄主互作过程中,病原菌通过分泌一系列细胞壁降解酶降解寄主植物的细胞壁,进而将多糖等营养物质从植物细胞内释放供病原菌生长,促进病原菌的侵入和定殖;多聚半乳糖醛酸酶已被鉴定为尖孢镰孢菌古巴专化型4号生理小种的致病因子(董章勇和王振中 2012).已有报道表明,FoSlt2 MAPK信号转导通路调控尖孢镰孢菌古巴专化型的生理特性和致病性(Dinget al. 2015 ;Dinget al. 2018a ;丁兆建等 2019b;Dinget al. 2020 );cAMP-PKA信号通路G蛋白亚基FGA2和FGB1参与调控尖孢镰孢菌古巴专化型的生长发育、产孢、耐热性和致病性(Guoet al. 2016 ).镰孢菌酸(fusaric acid,FA)是镰孢菌产生的非寄主专化性毒素,对动物和人类具有中低毒性,但对植物有较高的毒性,可引起多种作物的萎蔫病和根腐病害(Brownet al. 2012 ;Niehauset al. 2014 ).国内外已报道尖孢镰孢菌古巴专化型的多个致萎相关毒素,包括FA、白僵菌素和伏马菌素B1(许文耀等 2004;陈石等 2011;李春雨等 2011;Ding et al. 2018b ;Portal et al. 2018 ).关于植物病原真菌致病相关sRNAs报道较多,灰霉菌Botrytis cinerea、大丽轮枝菌Verticillium dahlia、小麦柄锈菌Puccinia striiformis f. sp. tritici和卵菌Oomycetes产生的sRNAs效应子可跨界传输到寄主细胞中,通过沉默寄主相关免疫基因,抑制寄主植物的免疫反应(Weiberget al. 2013 ;Wanget al. 2016 ;Wanget al. 2017 );关于尖孢镰孢菌古巴专化型sRNAs的研究处于起步阶段,李敏慧等(2019)发现用该病菌接种香蕉苗根部24h后,病原菌编码Dicer 和Argonaute的基因表达显著升高,sRNA测序结果也表明,大量milRNA显著高表达.也有多个相关致病基因得到鉴定,如活性氧迸发转录因子Foatf1(Qiet al. 2013 )、编码α-1,6-甘露糖转移酶的基因FoOCH1(Liet al. 2014 )、微管蛋白β1和β2(刘远征等 2018a,2018b)和Ⅴ型肌球蛋白Myosin5的协作蛋白smy1(何壮等 2019).尽管该菌的致病机理研究已取得了显著进展,但其致病机制仍不清晰,尤其对作为初侵染源的厚垣孢子的研究也尚未引起足够的重视. ...
香蕉枯萎病菌新毒素——白僵菌素的鉴定
1
2011
... 尖孢镰孢菌古巴专化型的致病因子主要包括细胞壁降解酶、信号转导通路、毒素、sRNAs和致病相关基因.在病原菌和寄主互作过程中,病原菌通过分泌一系列细胞壁降解酶降解寄主植物的细胞壁,进而将多糖等营养物质从植物细胞内释放供病原菌生长,促进病原菌的侵入和定殖;多聚半乳糖醛酸酶已被鉴定为尖孢镰孢菌古巴专化型4号生理小种的致病因子(董章勇和王振中 2012).已有报道表明,FoSlt2 MAPK信号转导通路调控尖孢镰孢菌古巴专化型的生理特性和致病性(Dinget al. 2015 ;Dinget al. 2018a ;丁兆建等 2019b;Dinget al. 2020 );cAMP-PKA信号通路G蛋白亚基FGA2和FGB1参与调控尖孢镰孢菌古巴专化型的生长发育、产孢、耐热性和致病性(Guoet al. 2016 ).镰孢菌酸(fusaric acid,FA)是镰孢菌产生的非寄主专化性毒素,对动物和人类具有中低毒性,但对植物有较高的毒性,可引起多种作物的萎蔫病和根腐病害(Brownet al. 2012 ;Niehauset al. 2014 ).国内外已报道尖孢镰孢菌古巴专化型的多个致萎相关毒素,包括FA、白僵菌素和伏马菌素B1(许文耀等 2004;陈石等 2011;李春雨等 2011;Ding et al. 2018b ;Portal et al. 2018 ).关于植物病原真菌致病相关sRNAs报道较多,灰霉菌Botrytis cinerea、大丽轮枝菌Verticillium dahlia、小麦柄锈菌Puccinia striiformis f. sp. tritici和卵菌Oomycetes产生的sRNAs效应子可跨界传输到寄主细胞中,通过沉默寄主相关免疫基因,抑制寄主植物的免疫反应(Weiberget al. 2013 ;Wanget al. 2016 ;Wanget al. 2017 );关于尖孢镰孢菌古巴专化型sRNAs的研究处于起步阶段,李敏慧等(2019)发现用该病菌接种香蕉苗根部24h后,病原菌编码Dicer 和Argonaute的基因表达显著升高,sRNA测序结果也表明,大量milRNA显著高表达.也有多个相关致病基因得到鉴定,如活性氧迸发转录因子Foatf1(Qiet al. 2013 )、编码α-1,6-甘露糖转移酶的基因FoOCH1(Liet al. 2014 )、微管蛋白β1和β2(刘远征等 2018a,2018b)和Ⅴ型肌球蛋白Myosin5的协作蛋白smy1(何壮等 2019).尽管该菌的致病机理研究已取得了显著进展,但其致病机制仍不清晰,尤其对作为初侵染源的厚垣孢子的研究也尚未引起足够的重视. ...
香蕉枯萎病菌4号生理小种β1-微管蛋白基因的功能分析
1
2018a
... 尖孢镰孢菌古巴专化型的致病因子主要包括细胞壁降解酶、信号转导通路、毒素、sRNAs和致病相关基因.在病原菌和寄主互作过程中,病原菌通过分泌一系列细胞壁降解酶降解寄主植物的细胞壁,进而将多糖等营养物质从植物细胞内释放供病原菌生长,促进病原菌的侵入和定殖;多聚半乳糖醛酸酶已被鉴定为尖孢镰孢菌古巴专化型4号生理小种的致病因子(董章勇和王振中 2012).已有报道表明,FoSlt2 MAPK信号转导通路调控尖孢镰孢菌古巴专化型的生理特性和致病性(Dinget al. 2015 ;Dinget al. 2018a ;丁兆建等 2019b;Dinget al. 2020 );cAMP-PKA信号通路G蛋白亚基FGA2和FGB1参与调控尖孢镰孢菌古巴专化型的生长发育、产孢、耐热性和致病性(Guoet al. 2016 ).镰孢菌酸(fusaric acid,FA)是镰孢菌产生的非寄主专化性毒素,对动物和人类具有中低毒性,但对植物有较高的毒性,可引起多种作物的萎蔫病和根腐病害(Brownet al. 2012 ;Niehauset al. 2014 ).国内外已报道尖孢镰孢菌古巴专化型的多个致萎相关毒素,包括FA、白僵菌素和伏马菌素B1(许文耀等 2004;陈石等 2011;李春雨等 2011;Ding et al. 2018b ;Portal et al. 2018 ).关于植物病原真菌致病相关sRNAs报道较多,灰霉菌Botrytis cinerea、大丽轮枝菌Verticillium dahlia、小麦柄锈菌Puccinia striiformis f. sp. tritici和卵菌Oomycetes产生的sRNAs效应子可跨界传输到寄主细胞中,通过沉默寄主相关免疫基因,抑制寄主植物的免疫反应(Weiberget al. 2013 ;Wanget al. 2016 ;Wanget al. 2017 );关于尖孢镰孢菌古巴专化型sRNAs的研究处于起步阶段,李敏慧等(2019)发现用该病菌接种香蕉苗根部24h后,病原菌编码Dicer 和Argonaute的基因表达显著升高,sRNA测序结果也表明,大量milRNA显著高表达.也有多个相关致病基因得到鉴定,如活性氧迸发转录因子Foatf1(Qiet al. 2013 )、编码α-1,6-甘露糖转移酶的基因FoOCH1(Liet al. 2014 )、微管蛋白β1和β2(刘远征等 2018a,2018b)和Ⅴ型肌球蛋白Myosin5的协作蛋白smy1(何壮等 2019).尽管该菌的致病机理研究已取得了显著进展,但其致病机制仍不清晰,尤其对作为初侵染源的厚垣孢子的研究也尚未引起足够的重视. ...
香蕉枯萎病菌4号生理小种β2-微管蛋白基因敲除与表型分析
1
2018b
... 尖孢镰孢菌古巴专化型的致病因子主要包括细胞壁降解酶、信号转导通路、毒素、sRNAs和致病相关基因.在病原菌和寄主互作过程中,病原菌通过分泌一系列细胞壁降解酶降解寄主植物的细胞壁,进而将多糖等营养物质从植物细胞内释放供病原菌生长,促进病原菌的侵入和定殖;多聚半乳糖醛酸酶已被鉴定为尖孢镰孢菌古巴专化型4号生理小种的致病因子(董章勇和王振中 2012).已有报道表明,FoSlt2 MAPK信号转导通路调控尖孢镰孢菌古巴专化型的生理特性和致病性(Dinget al. 2015 ;Dinget al. 2018a ;丁兆建等 2019b;Dinget al. 2020 );cAMP-PKA信号通路G蛋白亚基FGA2和FGB1参与调控尖孢镰孢菌古巴专化型的生长发育、产孢、耐热性和致病性(Guoet al. 2016 ).镰孢菌酸(fusaric acid,FA)是镰孢菌产生的非寄主专化性毒素,对动物和人类具有中低毒性,但对植物有较高的毒性,可引起多种作物的萎蔫病和根腐病害(Brownet al. 2012 ;Niehauset al. 2014 ).国内外已报道尖孢镰孢菌古巴专化型的多个致萎相关毒素,包括FA、白僵菌素和伏马菌素B1(许文耀等 2004;陈石等 2011;李春雨等 2011;Ding et al. 2018b ;Portal et al. 2018 ).关于植物病原真菌致病相关sRNAs报道较多,灰霉菌Botrytis cinerea、大丽轮枝菌Verticillium dahlia、小麦柄锈菌Puccinia striiformis f. sp. tritici和卵菌Oomycetes产生的sRNAs效应子可跨界传输到寄主细胞中,通过沉默寄主相关免疫基因,抑制寄主植物的免疫反应(Weiberget al. 2013 ;Wanget al. 2016 ;Wanget al. 2017 );关于尖孢镰孢菌古巴专化型sRNAs的研究处于起步阶段,李敏慧等(2019)发现用该病菌接种香蕉苗根部24h后,病原菌编码Dicer 和Argonaute的基因表达显著升高,sRNA测序结果也表明,大量milRNA显著高表达.也有多个相关致病基因得到鉴定,如活性氧迸发转录因子Foatf1(Qiet al. 2013 )、编码α-1,6-甘露糖转移酶的基因FoOCH1(Liet al. 2014 )、微管蛋白β1和β2(刘远征等 2018a,2018b)和Ⅴ型肌球蛋白Myosin5的协作蛋白smy1(何壮等 2019).尽管该菌的致病机理研究已取得了显著进展,但其致病机制仍不清晰,尤其对作为初侵染源的厚垣孢子的研究也尚未引起足够的重视. ...
香蕉枯萎病菌粗毒素的毒性及其模型
1
2004
... 尖孢镰孢菌古巴专化型的致病因子主要包括细胞壁降解酶、信号转导通路、毒素、sRNAs和致病相关基因.在病原菌和寄主互作过程中,病原菌通过分泌一系列细胞壁降解酶降解寄主植物的细胞壁,进而将多糖等营养物质从植物细胞内释放供病原菌生长,促进病原菌的侵入和定殖;多聚半乳糖醛酸酶已被鉴定为尖孢镰孢菌古巴专化型4号生理小种的致病因子(董章勇和王振中 2012).已有报道表明,FoSlt2 MAPK信号转导通路调控尖孢镰孢菌古巴专化型的生理特性和致病性(Dinget al. 2015 ;Dinget al. 2018a ;丁兆建等 2019b;Dinget al. 2020 );cAMP-PKA信号通路G蛋白亚基FGA2和FGB1参与调控尖孢镰孢菌古巴专化型的生长发育、产孢、耐热性和致病性(Guoet al. 2016 ).镰孢菌酸(fusaric acid,FA)是镰孢菌产生的非寄主专化性毒素,对动物和人类具有中低毒性,但对植物有较高的毒性,可引起多种作物的萎蔫病和根腐病害(Brownet al. 2012 ;Niehauset al. 2014 ).国内外已报道尖孢镰孢菌古巴专化型的多个致萎相关毒素,包括FA、白僵菌素和伏马菌素B1(许文耀等 2004;陈石等 2011;李春雨等 2011;Ding et al. 2018b ;Portal et al. 2018 ).关于植物病原真菌致病相关sRNAs报道较多,灰霉菌Botrytis cinerea、大丽轮枝菌Verticillium dahlia、小麦柄锈菌Puccinia striiformis f. sp. tritici和卵菌Oomycetes产生的sRNAs效应子可跨界传输到寄主细胞中,通过沉默寄主相关免疫基因,抑制寄主植物的免疫反应(Weiberget al. 2013 ;Wanget al. 2016 ;Wanget al. 2017 );关于尖孢镰孢菌古巴专化型sRNAs的研究处于起步阶段,李敏慧等(2019)发现用该病菌接种香蕉苗根部24h后,病原菌编码Dicer 和Argonaute的基因表达显著升高,sRNA测序结果也表明,大量milRNA显著高表达.也有多个相关致病基因得到鉴定,如活性氧迸发转录因子Foatf1(Qiet al. 2013 )、编码α-1,6-甘露糖转移酶的基因FoOCH1(Liet al. 2014 )、微管蛋白β1和β2(刘远征等 2018a,2018b)和Ⅴ型肌球蛋白Myosin5的协作蛋白smy1(何壮等 2019).尽管该菌的致病机理研究已取得了显著进展,但其致病机制仍不清晰,尤其对作为初侵染源的厚垣孢子的研究也尚未引起足够的重视. ...