羊肚菌Morchella隶属于子囊菌门Ascomycota、盘菌纲Pezizomycetes、盘菌目Pezizales、羊肚菌科Morchellaceae(杜习慧等 2014),其味道鲜美,肉质脆嫩可口,风味独特,在欧洲仅次于块菌,是食(药)用菌中的珍品之一,自古以来一直被奉为宴席上的美味佳肴(杜习慧等 2014)。羊肚菌营养丰富,含有多糖、生物酶类、氨基酸、脂肪酸,还有维生素和钙、锌、铁等多种矿物质(Gençcelep et al. 2009;刘蓓等 2012)。现代医学研究表明,羊肚菌有调节机体免疫力、抗疲劳、抑制肿瘤、抗菌、抗病毒、降血脂、抗氧化等多种生物活性(Mau et al. 2004;Turkoglu et al. 2006;Gursoy et al. 2009;殷伟伟等 2009;Ozturk et al. 2010;罗霞等 2011;王亚辉等 2013;马利等 2014;Wu et al. 2019)。羊肚菌在国内外市场上备受欢迎,日本、美国、欧洲、韩国、东南亚以及我国都有很大需求。
六妹羊肚菌Morchella sextelata M. Kuo属于黑色羊肚菌支系Elata Clade(杜习慧等 2014),是近年来我国羊肚菌大田栽培模式下成功驯化的羊肚菌品种,具有明显的抗极端气候特征,且产量稳定,是四川、湖北和贵州等地的主推品种(郜惠苹等 2020)。目前,针对六妹羊肚菌的研究多集中于栽培和多糖成分(熊川等 2017;Meng et al. 2019;王珍珍等 2019;郜惠苹等 2020),未见小分子化学成分的研究报道。有鉴于此,本研究以六妹羊肚菌子实体为对象,开展了两方面的化学成分研究工作:1)采用气相色谱-质谱联用技术,对其芳香物和亲脂性提取物的化学成分进行了分析,并对亲脂性提取物的生物活性进行初探;2)采用现代色谱分离技术并结合各种结构鉴定方法,对其化学成分进行分离和结构鉴定。
1 材料与方法
1.1 供试材料
1.1.1 六妹羊肚菌子实体:成熟的六妹羊肚菌子实体采自四川成都新都区羊肚菌种植基地,菌种由四川省农业科学院土壤肥料研究所唐杰副研究员鉴定。
1.1.2 试剂:2,2-二(4-叔辛基苯基)-1-苦肼基自由基[2,2-Di(4-tert-octylphenyl)-1-picrylhydrazyl, free radical, DPPH]和2,2’-联氮基-双-(3-乙基苯并二氢噻唑啉-6-磺酸)二铵盐[2,2’-Azino- bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt,ABTS]均购自美国Sigma- Aldrich公司,十八烷基键合硅胶(ODS)(孔径120Å,粒径50μm,日本YMC公司),葡聚糖凝胶(Sephadex LH-20)(美国GE Healthcare公司),硅胶(100-200目,200-300目,硅胶H,硅胶GF254,青岛海洋化工厂),其他试剂为分析纯(成都科隆化学品有限公司)。
1.1.3 仪器:QP2010 Plus气相色谱-质谱联用仪(日本岛津),SH-Rxi-5MS毛细管色谱柱(30m×0.25mm i.d.,涂层厚0.25μm,日本岛津),AV II-600和400核磁共振仪(TMS作为内标,美国Bruker公司),Q-TOF Premier高分辨质谱(美国Waters公司),550型酶标仪(美国BIO-RAD),X-4显微熔点仪(上海易测仪器设备有限公司,未校订)。
1.2 亲脂性提取物的提取
采用低极性溶剂浸提法进行六妹羊肚菌子实体中亲脂性提取物的提取(Kowalski 2011)。将新鲜六妹羊肚菌子实体切成小块(1.0cm±0.5cm),转移至带盖烧瓶中,加入重蒸石油醚(沸程30-60℃),烧瓶室温下避光静置7d。分离出石油醚提取液,无水Na2SO4干燥后减压蒸馏回收溶剂得到亲脂性提取物。亲脂性提取物放置于冰箱中(4℃)备用。
1.3 芳香物的提取
采用顶空固相微萃取法(HS-SPME)进行六妹羊肚菌子实体中芳香物的提取(Mateie et al. 2019)。新鲜六妹羊肚菌子实体经破碎后,称量2.5g放入20mL顶空瓶内密封。将已经老化好的萃取头(50/30μm,DVB/CAR/PDMS,美国Supulco公司)插入装有样品的顶空瓶中,萃取针位于样品液面上,在恒温(50℃)槽内萃取45min。从顶空瓶内取出萃取完成的萃取头,插入GC-MS进样口(进样口温度270℃),在270℃下解吸附1min,供GC-MS分析。
1.4 气相色谱-质谱分析(GC-MS)
1.4.1 芳香物分析的气相色谱条件:程序升温:从40℃开始,保持5min,然后以5℃/min升到160℃,保持2min,随后再以10℃/min升到280℃,保持5min;进样口温度为270℃;载气为氦气;柱流量:1mL/min;分流比:5:1。
1.4.2 亲脂性提取物分析的气相色谱条件:程序升温:从40℃开始,保持5min,然后以10℃/min升到220℃,保持2min,随后再以5℃/min升到290℃,保持20min;进样口温度为290℃;载气为氦气;柱流量:1mL/min;进样量为1μL;分流比:5:1。
1.4.3 质谱条件:EI离子源;电离电压:70eV;扫描范围:33-600amu;离子源温度:200℃。样品中各成分通过其质谱与标准质谱数据库(NIST08.LIB)检索比对确认。采用峰面积归一化方法确认各组分的相对含量(%)。
1.5 化学成分的提取与分离
将9kg干燥的六妹羊肚菌子实体粉碎后,用95%乙醇室温浸提3次(45L/次,7d/次),合并乙醇提取液后减压浓缩至无醇味,得到乙醇提取物1.6L。往乙醇提取物中加入8L纯净水进行稀释后,用乙酸乙酯萃取3次(10L/次),合并乙酸乙酯萃取液,减压浓缩回收溶剂后得到黑褐色乙酸乙酯浸膏165g。
乙酸乙酯浸膏(110g)经硅胶柱色谱(200-300目,2.2kg),石油醚-乙酸乙酯(体积比:100:1-1:1)梯度洗脱,得22个组分(Fr.1-22)。Fr.4(1g)经硅胶柱色谱(环己烷-丙酮=100:1-60:1)得到5个组分Fr.4.1-4.5,Fr.4.1(108mg)经葡聚糖凝胶柱色谱(Sephadax LH-20,氯仿-甲醇=2:1,下同)得到化合物1(12mg,无色油状物),Fr.4.2(300mg)经硅胶柱色谱(环己烷-丙酮=100:1)得两个组分Fr.4.2.1和Fr.4.2.2,。Fr.4.2.2(278mg)经葡聚糖凝胶柱色谱纯化得化合物2(41mg,无色油状物),Fr.4.4(70.6mg)经葡聚糖凝胶柱色谱纯化得到化合物3(26mg,无色油状物)。Fr.7(1.1g)经硅胶柱色谱(环己烷-乙酸乙酯=60:1)得5个组分Fr.7.1-7.5,Fr.7.2(54mg)经葡聚糖凝胶柱色谱纯化得化合物4(8mg,无色针状晶体,乙酸乙酯)。Fr.11(2.2g)以石油醚为溶剂进行重结晶得化合物5(57mg,无色针状晶体)。Fr.14(3g)以石油醚-乙酸乙酯混合溶剂进行重结晶得化合物6(305mg,无色针状晶体)。Fr.15(550mg)经硅胶柱色谱(石油醚-乙酸乙酯=50:1)得4个组分Fr.12.1-12.4,Fr.15.2(149mg)分别经葡聚糖凝胶柱色谱和ODS反相硅胶柱色谱(甲醇-水=17:3)得化合物7(5mg,白色无定型粉末)。Fr.16(1.48g)经硅胶柱色谱(石油醚-乙酸乙酯=50:1)得到6个组分Fr.16.1-16.6,Fr.16.1(244mg)分别经葡聚糖凝胶柱色谱和ODS反相硅胶柱色谱(甲醇)得到化合物8(41mg,无色油状物)。Fr.17以石油醚-丙酮混合溶剂进行重结晶到化合物9(无色透明针状晶体),剩余物(1.5g)经硅胶柱色谱(石油醚-乙酸乙酯=30:1)得到4个组分Fr.17.1-17.4,Fr.17.2(320mg)经乙酸乙酯重结晶后得到化合物10(26mg,无色针状晶体),Fr.17.4(167mg)经硅胶柱色谱(石油醚-乙酸乙酯=50:1)和葡聚糖凝胶柱色谱纯化得化合物11(4mg,无色针状晶体,氯仿-甲醇)。Fr.18经过滤除去固体物后滤液浓缩得残余物532mg,经硅胶柱色谱(石油醚-乙酸乙酯=3:1)得到两个组分Fr.18.1-18.2,Fr.18.2(73mg)经过葡聚糖凝胶柱色谱纯化得到化合物12(27mg,无定型粉末)。Fr.19(1.25g)经硅胶柱色谱(石油醚-乙酸乙酯=10:1)得到5个组分Fr.19.1-19.5,Fr.19.2经葡聚糖凝胶柱色谱,ODS反相硅胶柱色谱(甲醇)得到化合物13(5mg,无色油状物),Fr.19.5经葡聚糖凝胶柱色谱,ODS反相硅胶柱色谱(甲醇)得到化合物14(4mg,无色针状晶体,甲醇)。
1.6 亲脂性提取物生物活性检测
1.6.1 抗氧化活性检测:DPPH自由基清除活性的检测参考Brand-Williams et al.(1995)的方法。样品以乙醇溶解后,采用两倍稀释法配成系列浓度的样品溶液(0.625-40mg/mL)。分别取不同浓度的样品溶液50μL,与DPPH溶液(0.1mg/mL)100μL混合,混合溶液室温下避光放置25min后,采用酶标仪于517nm下测定吸光度值。以维生素C为阳性对照,以不含样品的同体积乙醇溶液为阴性对照,所有实验重复3次。自由基清除率(%)=[(A对照- (A样品-A空白))/A对照]×100,其中A对照为阴性对照的吸光度值,A样品为样品的吸光度值,A空白为不含DPPH的混合溶液吸光度值,自由基清除率达50%的有效浓度(EC50)通过SPSS软件计算。
ABTS自由基清除活性的检测参考Re et al.(1999)的方法。将ABTS溶液(7mmol/L)和过硫酸钾溶液(2.45mmol/L)混合后,用乙醇稀释至混合溶液吸光度值达到0.6-0.7(734nm),制得ABTS自由基溶液备用。样品以乙醇溶解后,仍采用两倍稀释法配成系列浓度的样品溶液(0.625-40mg/mL)。分别取不同浓度的样品溶液50μL,与ABTS自由基溶液100μL混合,混合溶液室温下避光放置5min后,采用酶标仪于734nm下测定吸光度值。以维生素C为阳性对照,以不含样品的同体积乙醇溶液为阴性对照,所有实验重复3次。自由基清除率参考上面公式计算。
1.6.2 抗菌活性检测:大肠杆菌Escherichia coli(ATCC 25922)、金黄色葡萄球菌Staphylococcus aureus(ATCC 25923)、枯草芽孢杆菌Bacillus subtilis(ATCC 6633)和白色念珠菌Candida albicans(ATCC 10231)被用于抗菌活性检测,参考Tu et al.(2021)的方法。样品以DMSO溶液后,采用两倍稀释法配置系列浓度的样品溶液(0.5-512μg/mL)。将不同浓度的样品溶液与菌液混合于96孔板的小孔中,细菌于37℃孵育24h,真菌于28℃孵育4d。以卡那霉素(针对细菌)和制霉菌素(针对真菌)为阳性对照,以不含样品的等体积DMSO为阴性对照,每次操作重复两次。以最小抑菌浓度(MIC)衡量样品的抗菌活性。
2 结果与分析
2.1 六妹羊肚菌的亲脂性提取物
因为采用水蒸气蒸馏法未能从六妹羊肚菌的子实体中获得挥发油,因此改用低级性溶剂浸提法,以期获得其亲脂性提取物。通过石油醚冷浸提取,从六妹羊肚菌新鲜子实体中获得的亲脂性提取物为淡黄色油状物,收率为0.116%(W/W,湿重)。
从亲脂性提取物中共鉴定出14个化合物,占总量的97.75%,包括2个醛类化合物、5个醇类化合物、4个羧酸/羧酸衍生物、3个甾体类化合物(表1)。其中,亚油酸为亲脂性提取物中的主要成分,占到总提取物的77.70%。亚油酸属于长链不饱和脂肪酸,具有重要的营养价值和生物活性,是人体必需脂肪酸。亚油酸有助于降低血清胆固醇水平,抑制动脉血栓的形成,在预防动脉粥样硬化和心肌梗塞等心血管疾病方面有良好作用(Marangoni et al. 2020)。近期亦有文献报道,亚油酸能在体外与SARS-CoV-2的刺突蛋白结合,阻碍其与ACE2相结合(Toelzer et al. 2020)。
表1 六妹羊肚菌子实体的芳香物和亲脂性提取物的化学组成
Table 1
| 化合物 Compounds | 亲脂性提取物 Lipophilic extracts (%) | 芳香物 Aromas (%) |
|---|---|---|
| 醛/酮类化合物 Aldehydes and Ketones | ||
| 乙醛 Acetaldehyde | - | 1.59 |
| 丙醛 Propanal | - | 0.92 |
| 3-甲基丁醛 3-Methyl-butanal | - | 7.28 |
| 2-甲基丙醛 2-Methyl-propanal | - | 0.92 |
| 2-甲基丁醛 2-Methyl-butanal | - | 3.82 |
| 己醛Hexanal | 0.08 | 1.62 |
| 庚醛 Heptanal | - | 0.05 |
| (Z)-庚-2-烯醛 (Z)-2-Heptenal | - | 0.08 |
| 苯甲醛 Benzaldehyde | - | 0.26 |
| 苯乙醛 Benzeneacetaldehyde | 0.07 | 12.31 |
| (E)-辛-2-烯醛 (E)-2-Octenal | - | 25.15 |
| 壬醛 Nonanal | - | 0.45 |
| (E)-壬-2-烯醛 (E)-2-Nonenal | - | 0.42 |
| 癸醛 Decanal | - | 0.27 |
| (E,E)-癸-2,4-二烯醛 (E,E)-2,4-Decadienal | - | 0.22 |
| (E,E)-辛-2,4-二烯醛 (E,E)-2,4-Octadienal | - | 0.21 |
| 辛-3-酮 3-Octanone | - | 1.32 |
| 醇类化合物 Alcohols | ||
| 3-甲基-丁-1-醇 3-Methyl-1-butanol | - | 0.25 |
| R-己-2-醇 R-2-Hexanol | 0.05 | |
| 辛-1-烯-3-醇 1-Octen-3-ol | 0.09 | 32.53 |
| 2-乙基己-1-醇2-Ethyl-1-hexanol | - | 0.20 |
| (E)-辛-2-烯-1-醇 (E)-2-Octen-1-ol | - | 4.16 |
| R-(-)-14-甲基十六-8-炔-1-醇 R-(-)-14-Methyl-8-hexadecyn-1-ol | 0.73 | - |
| (Z,Z)-十五-6,9-二烯-1-醇 (Z,Z)-6,9-Pentadecadien-1-ol | 0.10 | - |
| 香叶基香叶醇 Geranylgeraniol | 0.21 | - |
| 羧酸/羧酸衍生物 Carboxylic acids/Carboxylic acid derivatives | ||
| 十五酸 Pentadecanoic acid | 1.54 | - |
| 亚油酸 Linoleic acid | 77.80 | - |
| (Z,Z)-十八-9,12-二烯酸甲酯 (Z,Z)-9,12-Octadecadienoic acid methyl ester | 0.37 | - |
| 亚油酸甘油三酯 Trilinolein | 2.46 | - |
| 杂环/含硫化合物 Heterocycles/Sulfurs | ||
| 2-丁基呋喃 2-Butyl furan | - | 0.06 |
| 3-甲硫基丙醛 3-(Methylthio)-propanal | - | 0.54 |
| 甾体类化合物 Steroids | ||
| (3β, 22E)-麦角甾-5,22-二烯-3-醇 (3β, 22E)-Ergosta-5,22-dien-3-ol | 6.20 | - |
| 麦角甾醇 Ergosterol | 6.73 | - |
| 豆甾醇 Stigmasterol | 1.32 | - |
| 其他类 Others | ||
| 3,5,5-三甲基己-2-烯 3,5,5-Trimethyl-2-hexene | - | 1.02 |
| 3-乙基-2-甲基-戊-1-烯 3-Ethyl-2-methyl-1-pentene | - | 3.61 |
| 2,6,11-三甲基十二烷 2,6,11-Trimethyl-dodecane | - | 0.09 |
| 合计In total | 97.75 | 99.35 |
2.2 六妹羊肚菌的芳香物
从六妹羊肚菌新鲜子实体的芳香物中共鉴定出26个化合物,占到总量的99.35%,包括16个醛类化合物、1个酮类化合物、4个醇类化合物、1个杂环化合物、1个含硫化合物和3个其他类型化合物(表1)。其中,辛-1-烯-3-醇、(E)-辛-2-烯醛、苯乙醛和3-甲基丁醛为芳香物中的主要成分,分别占总量的32.53%、25.15%、12.31%和7.28%。
醛类化合物占总芳香物的55.57%,其中(E)-辛-2-烯醛(25.15%),苯乙醛(12.31%)和3-甲基丁醛(7.28%)为3个主要的醛类成分。食用菌挥发性芳香物中的醛类成分多具有不同的香味,(E)-辛-2-烯醛具有甜香味,苯乙醛具有花香味,3-甲基丁醛具有果香味(Cho et al. 2007;李琴等 2011)。醇类化合物占总芳香物的37.14%,其中辛-1-烯-3-醇(32.53%)和(E)-辛-2-烯-1-醇(4.16%)为主要的醇类成分。作为主要成分的辛-1-烯-3-醇具有蘑菇样的香味,常见于各种食用菌的芳香物中,是食用菌芳香物中的代表性成分(Cho et al. 2008;Culleré et al. 2013)。
2.3 化合物的结构鉴定
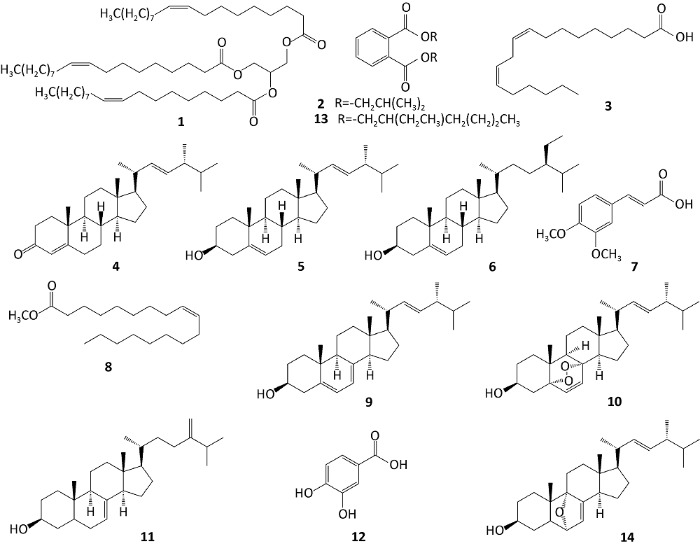
化合物1-14的结构见图1。
图1
化合物1,无色油状物;1H NMR (400MHz,CDCl3) δ: 5.34 (6H, m, H-9, 9’, 9’’, 10, 10’, 10’’), 5.27 (m, 1H, Glycerol H-2), 4.30 (2H, dd, J= 11.6, 4.4Hz, Glycerol H-1α, H-3α), 4.15 (2H, dd, J=11.6, 6.0Hz, Glycero H-1β, H-3β), 2.31 (6H, m, H-2, 2’, 2’’), 2.02 (12H, m, H-8, 8’, 8’’, 11, 11’, 11’’), 1.61 (6H, m, H-3, 3’, 3’’), 1.25-1.30 (60H, m, 3×10×CH2), 0.88 (9H, m, H-18, 18’, 18’’); 13C NMR (100MHz, CDCl3) δ: 173.3, 173.2, 172.8 (C-1, 1’, 1’’), 130.2, 130.0, 129.6 (C-9, 9’, 9’’), 128.0, 128.0, 127.9 (C-10, 10’, 10’’), 68.8 (Glycerol C-2), 62.1 (Glycerol C-1, 3), 34.2, 34.0, 34.0 (C-2, 2’, 2’), 31.9, 31.9, 31.5 (C-16, 16’, 16’’), 29.0-29.7 (3×9-CH2), 27.2, 27.2, 27.1 (C-8, 8’, 8’’), 25.6, 24.8, 24.8 (C-11, 11’, 11’’), 22.7, 22.7, 22.5 (C-17, 17’, 17’’), 14.2, 14.1, 14.0 (C-18, 18’, 18’’)。以上数据与Hamid et al.(2017)的报道一致,故鉴定为三油酸甘油酯(glycerol trioleate)。
化合物2,无色油状物;1H NMR (400MHz,CDCl3) δ: 7.73 (2H, m, H-3, 6), 7.53 (2H, m, H-4, 5), 4.10 (4H, d, J=6.8Hz, H-1’, 1’’), 2.04 (2H, m, H-2’, 2’’), 0.98 (12H, d, J=6.8Hz, H-3’, 3’’, 4’, 4’’); 13C NMR (100MHz, CDCl3) δ: 167.6 (2×C-α), 132.3 (C-1, 2), 130.9 (C-3, 6), 128.8 (C-4, 5), 71.7 (C-1’, 1’’), 27.7 (C-2’, 2’’), 19.1 (C-3’, 3’’, 4’, 4’’)。以上数据与韩冰洋等(2016)的报道一致,故鉴定为邻苯二甲酸二异丁酯(phthalic acid diisobutyl ester)。
化合物3,无色油状物;1H NMR (400MHz,CDCl3) δ: 5.34 (4H, m, H-9, 10, 12, 13), 2.77 (2H, dd, J=6.5Hz, H-11), 2.34 (2H, t, J=7.0Hz, H-2), 2.05 (4H, q, J=7.0Hz, H-8, 14), 1.63 (2H, m, H-3), 1.25-1.32 (14H, m, H-4, 5, 6, 7, 15, 16, 17), 0.88 (3H, m, H-18); 13C NMR (100MHz, CDCl3) δ: 179.5 (C-1), 130.2 (C-9), 130.0 (C-13), 128.0 (C-10), 127.9 (C-12), 34.0 (C-2), 31.5 (C-16), 31.5 (C-6), 29.7-29.1 (C-4, 5, 7, 15), 27.2, 27.2 (C-8, 14), 25.6 (C-3), 24.7 (C-11), 22.7 (C-17), 14.1 (C-18)。以上数据与Kim et al.(2016)的报道一致,故鉴定为亚油酸(linoleic acid)。
化合物4,无色针状晶体(乙酸乙酯);1H NMR (400MHz,CDCl3) δ: 5.72 (1H, s, H-4), 5.18 (2H, dd, J=10.0, 7.2Hz, H-22, 23), 1.18 (3H, s, H-19), 1.00 (3H, d, J=6.8Hz, H-21), 0.91 (3H, d, J=6.6Hz, H-28), 0.83 (3H, d, J=6.6Hz, H-26), 0.82 (3H, d, J=6.6Hz, H-27), 0.72 (3H, s, H-18); 13C NMR (100MHz, CDCl3) δ: 199.7 (C-3), 171.7 (C-5), 135.6 (C-22), 131.8 (C-23), 123.7 (C-4), 55.9 (C-17), 55.8 (C-14), 53.8 (C-9), 42.8 (C-24), 42.2 (C-13), 40.1 (C-20), 39.5 (C-12), 38.6 (C-1), 35.6 (C-10), 35.6 (C-8), 34.0 (C-2), 33.1 (C-25), 32.9 (C-6), 32.0 (C-7), 28.5 (C-16), 24.2 (C-15), 21.0 (C-11), 20.9 (C-21), 19.9 (C-26), 19.6 (C-27), 17.6 (C-28), 17.3 (C-19), 12.1 (C-18)。以上数据与Sun et al.(2012)的报道一致,故鉴定为ergosta-4,22-dien-3-one。
化合物5,无色针状晶体(石油醚);1H NMR (400MHz,CDCl3) δ: 5.35 (1H, d, J=5.2Hz, H-6), 5.18 (2H, m, H-22, 23), 3.49 (1H, m, H-3), 1.01 (3H, d, J=6.4Hz, H-28), 1.00 (3H, s, H-19), 0.91 (3H, d, J=6.8Hz, H-21), 0.83 (3H, d, J=6.4Hz, H-27), 0.81 (3H, d, J=6.5Hz, H-26), 0.69 (3H, s, H-18); 13C NMR (100MHz, CDCl3) δ: 140.7 (C-5), 135.8 (C-22), 131.7 (C-23), 121.7 (C-6), 70.8 (C-3), 56.8 (C-14), 56.0 (C-17), 50.1 (C-9), 42.8 (C-24), 42.3 (C-13), 42.1 (C-4), 40.1 (C-20), 39.6 (C-12), 37.2 (C-1), 36.5 (C-10), 33.7 (C-25), 33.1 (C-8), 31.6 (C-7), 30.5 (C-2), 28.5 (C-16), 24.3 (C-15), 21.0 (C-11), 20.9 (C-19), 19.9 (C-21), 19.6 (C-26), 19.4 (C-27), 17.6 (C-28), 12.0 (C-18)。以上数据与Gao et al. (2001)的报道一致,故鉴定为ergosta-5,22- dien-3β-ol。
化合物6,无色针状晶体(石油醚-乙酸乙酯);熔点:141-143℃;1H NMR (400MHz,CDCl3) δ: 5.35 (1H, m, H-6), 3.52 (1H, m, H-3), 1.00 (3H, s, H-19), 0.92 (3H, d, J=6.5Hz, H-21), 0.84 (3H, t, J=7.6Hz, H-29), 0.83 (3H,d, J=7.1Hz, H-26), 0.81 (3H, d, J=6.8Hz, H-27), 0.67 (3H, s, H-18)。将以上数据与Zhang et al.(2020)的报道进行比较,并将化合物6与β-谷甾醇标准品共薄层色谱对比(环己烷-乙酸乙酯3:1;环己烷-丙酮4:1;氯仿-乙酸乙酯10:1),结果显示Rf值一致,故鉴定为β-谷甾醇(β-sitosterol)。
化合物7,白色无定型粉末;熔点:180- 182℃;1H NMR (400MHz,CDCl3) δ: 7.75 (1H, d, J=15.0Hz, H-7), 7.14 (1H, d, J=6.4Hz, H-6), 7.08 (1H, s, H-2), 6.89 (1H, d, J=8.4Hz, H-5), 6.30 (1H, d, J=16.0Hz, H-8), 3.93 (6H, s, -OCH3)。通过将以上数据与费永和等(2014)的结果进行比较,并将化合物7与3,4-二甲氧基肉桂酸标准品共薄层色谱对比(石油醚-乙酸乙酯2:1;环己烷-丙酮4:1;氯仿-乙酸乙酯10:1,每个展开系统均滴加两滴甲酸),结果显示Rf值一致,故鉴定为3,4-二甲氧基肉桂酸(3,4-dimethoxycinnamic acid)。
化合物8,无色油状物;1H NMR (400MHz,CDCl3) δ: 5.34 (2H, m, H-9, 10), 3.67 (3H, s, -OCH3), 2.30 (2H, t, J=8.0Hz, H-2), 2.02 (4H, m, H-8, 11), 1.62 (2H, m, H-3), 1.25-1.32 (20H, m, 10×CH2), 0.88 (3H, t, J=8.0Hz, H-18); 13C NMR (100MHz, CDCl3) δ: 174.3 (C-1), 130.0 (C-9), 129.7 (C-10), 51.4 (-OCH3), 34.1 (C-2), 29.7- 29.1 (9×CH2), 27.2, 27.1 (C-8, 11), 24.9 (C-3), 22.7 (C-17), 14.1 (C-18)。以上数据与麻兵继和王佩佩(2008)的报道一致,故鉴定为油酸甲酯(methyl oleate)。
化合物9,无色针状结晶(石油醚-丙酮);1H NMR (400MHz,CDCl3) δ: 5.57 (1H, s, H-6), 5.39 (1H, s, H-7), 5.19 (2H, m, H-22, 23), 3.64 (1H, m, H-3), 1.03 (3H, d, J=6.4Hz, H-21), 0.94 (3H, s, H-19), 0.91 (3H, d, J=7.5Hz, H-28), 0.84 (3H, d, J=6.4Hz, H-26), 0.82 (3H, d, J=6.4Hz, H-27), 0.63 (3H, s, H-18); 13C NMR (100MHz, CDCl3) δ: 141.3 (C-8), 139.8 (C-5), 135.5 (C-22), 131.9 (C-23), 119.5 (C-6), 116.3 (C-7), 70.4 (C-3), 55.7 (C-17), 54.5 (C-14), 46.2 (C-9), 42.8 (C-13), 42.8 (C-24), 40.8 (C-4), 40.4 (C-20), 39.1 (C-12), 38.4 (C-1), 37.0 (C-10), 33.1 (C-25), 32.0 (C-2), 28.31 (C-16), 23.0 (C-15), 21.1 (C-11), 21.0 (C-21), 19.9, 19.6 (C-26, 27), 17.6 (C-28), 16.2 (C-19), 12.0 (C-18)。以上数据与Shirane et al.(1996)的报道一致,故鉴定为麦角甾醇(ergosterol)。
化合物10,无色针状结晶(乙酸乙酯);熔点:176-178℃;1H NMR (600MHz,CDCl3) δ: 6.50 (1H, d, J=9.0Hz, H-7), 6.25 (1H, d, J=8.4Hz, H-6), 5.20 (1H, dd, J=15.0Hz, 7.8Hz, H-23), 5.15 (1H, dd, J=15.0Hz, 8.4Hz, H-22), 3.97 (1H, m, H-3), 1.00 (3H, d, J=6.6Hz, H-21), 0.90 (3H, d, J=6.7Hz, H-28), 0.88 (3H, s, H-19), 0.83 (3H, d, J=6.8Hz, H-27), 0.81 (3H, d, J=6.6Hz, H-26), 0.81 (3H, s, H-18)。通过将以上数据与Zhang et al.(2020)的结果进行比较,并将化合物10与过氧麦角甾醇标准品共薄层色谱对比(石油醚-乙酸乙酯2:1;环己烷-丙酮3:1;氯仿-甲醇7:1),结果显示Rf值一致,故鉴定为过氧麦角甾醇(5α,8α- peroxyergosterol)。
化合物11,无色针状结晶(氯仿-甲醇);1H NMR (400MHz,CDCl3) δ: 5.16 (1H, m, H-7), 4.71, 4.65 (2H, br s, H-28), 4.65 (1H, br s, H-28), 3.59 (1H, m, H-3), 2.23 (1H, m, H-25), 1.02 (3H, d, J=6.8Hz, H-26), 1.02 (3H, d, J=6.8Hz, H-27), 0.95 (3H, d, J=6.5Hz, H-28), 0.79 (3H, s, H-19), 0.54 (3H, s, H-18); 13C NMR (100MHz, CDCl3) δ: 156.8 (C-24), 139.5 (C-8), 117.5 (C-7), 105.9 (C-28), 71.0 (C-3), 56.0 (C-17), 55.0 (C-14), 49.4 (C-9), 43.4 (C-13), 40.2 (C-5), 39.5 (C-12), 37.9 (C-4), 37.1 (C-1), 36.2 (C-20), 34.6 (C-22), 34.2 (C-10), 33.8 (C-25), 31.9 (C-2), 31.0 (C-23), 29.7 (C-6), 27.9 (C-16), 22.9 (C-15), 22.0 (C-26), 21.8 (C-27), 21.5 (C-11), 18.8 (C-21), 13.8 (C-19), 11.8 (C-18)。以上数据与Shirane et al.(1996)的报道一致,故鉴定为麦角甾-7,24(28)-二烯-3β-醇(ergosta- 7,24(28)-dien-3β-ol)。
化合物12,无定型粉末;1H NMR (400MHz,CD3OD) δ: 7.46 (1H, br s, H-2), 7.45 (1H, dd, J=7.8, 2.0Hz, H-6), 6.82 (1H, d, J=8.4Hz, H-5); 13C NMR (100MHz, CDCl3) δ: 168.9 (C=O), 150.1 (C-4), 144.6 (C-3), 122.5 (C-6), 121.6 (C-1), 116.3 (C-2), 114.4 (C-5)。以上数据与Zhang et al.(1998)的报道一致,故鉴定为原儿茶酸(protocatechuic acid)。
化合物13,无色油状物;1H NMR (400MHz,CDCl3) δ: 7.73 (2H, m, H-3’’, 6‘’), 7.52 (2H, m, H-4’’, 5’’), 4.22 (4H, m, H-1, 1’), 1.69 (2H, m, H-2, 2’), 1.42 (16H, m, H-3, 3’, 4, 4’, 5, 5’, 7, 7’), 0.98 (12H, m, H-6, 6’, 8, 8’); 13C NMR (100MHz, CDCl3) δ: 167.7 (2×C=O), 132.4 (C-1’’, 2’’), 130.9 (C-3’, 6’), 128.8 (C-4’’, 5’’), 68.1 (C-1, 1’), 38.7 (C-2, 2’), 30.3 (C-3, 3’), 28.9 (C-4, 4’), 23.7 (C-7, 7’), 23.0 (C-5, 5’), 14.0 (C-8, 8’), 10.9 (C-6, 6’)。以上数据与Katade et al.(2006)的报道一致,故鉴定为邻苯二甲酸二(2-乙基)己酯[bis(2-ethylhexyl) benzene-1,2- dicarboxylate]。
化合物14,无色针状晶体(甲醇);HR-ESI-MS [M-H]- m/z 411.3282 (calcd. for C28H43O2, 411.3263);1H NMR (600MHz,CDCl3/CD3OD) δ: 5.31 (1H, s, H-7), 5.18 (2H, m, H-22, 23), 3.98 (1H, m, H-3), 3.57 (1H, d, J=4.8Hz, H-6), 1.07 (3H, s, H-19), 1.03 (3H, d, J=6.6Hz, H-21), 0.92 (3H, d, J=6.6Hz, H-28), 0.84 (3H, d, J=6.8Hz, H-26), 0.82 (3H, d, J=3.7Hz, H-27), 0.62 (3H, s, H-18); 13C NMR (100MHz, CDCl3/CD3OD) δ: 143.4 (C-8), 135.5 (C-22),131.9 (C-23), 114.7 (C-7), 75.9 (C-9), 72.7 (C-6), 67.0 (C-3), 55.9 (C-17), 54.7 (C-14), 43.5 (C-13),43.3 (C-10), 42.8 (C-24), 40.4 (C-20), 39.2 (C-4), 39.2 (C-12), 37.0 (C-5), 33.0 (C-25), 32.4 (C-1), 30.2 (C-2), 27.8 (C-16), 22.8 (C-11), 21.8 (C-15), 20.8 (C-21), 19.6 (C-27), 19.2 (C-26), 17.8 (C-19), 17.3 (C-28), 11.9 (C-18)。以上数据与Guo et al.(2007)的报道一致,故鉴定为6,9-epoxy-ergosta-7,22-dien-3β-ol。
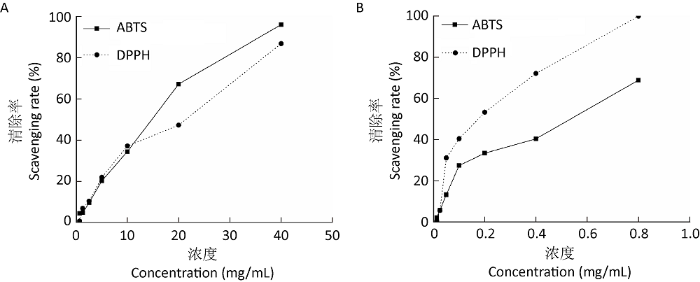
2.4 六妹羊肚菌亲脂性提取物的生物活性
图2
图2
六妹羊肚菌亲脂性提取物清除自由基能力曲线
A:亲脂性提取物;B:维生素C
Fig. 2
Radical scavenging activities of lipophilic extracts from Morchella sextelata.
A: Lipophilic extracts; B: Vitamin C.
2.4.2 抗菌活性:本研究共检测了六妹羊肚菌亲脂性提取物对4种微生物的抑菌活性,包括属于革兰氏阳性菌的金黄色葡萄球菌和枯草芽孢杆菌,属于革兰氏阴性菌的大肠杆菌,属于真菌的白色念珠菌(表2)。结果显示,相较于阳性对照卡那霉素和制霉菌素,六妹羊肚菌亲脂性提取物仅对枯草芽孢杆菌显示出微弱的抑菌活性(MIC 32μg/mL),对其他微生物没有明显的抗菌活性(MIC ≥512μg/mL)。
表2 六妹羊肚菌亲脂性提取物的抗菌活性
Table 2
| 样品 Samples | 大肠杆菌 Escherichia coli | 金黄色葡萄球菌 Staphylococcus aureus | 枯草芽孢杆菌 Bacillus subtilis | 白色念珠菌 Candida albicans |
|---|---|---|---|---|
| 亲脂性提取物 Lipophilic extracts | 512 | 512 | 32 | 512 |
| 卡那霉素 Kanamycin | 1 | 1 | <1 | - |
| 制霉菌素 Nystatin | - | - | - | 1 |
3 讨论
六妹羊肚菌作为一种大田栽培模式下人工种植的羊肚菌品种,在我国广泛种植。本研究首次对六妹羊肚菌子实体的小分子化合物进行了分析。
六妹羊肚菌子实体的亲脂性提取物中化学成分以亚油酸为主,占总量的77.70%,其化学组成与另一人工种植羊肚菌品种——梯棱羊肚菌M. importuna的亲脂性提取物相似(Tu et al. 2021)。亚油酸的大量存在很好地体现了羊肚菌的营养价值。六妹羊肚菌子实体芳香物的化学成分多样,包含了醛、酮、醇、杂环、含硫化合物、长链烷烃和烯烃
等,其化学组成和主要成分与梯棱羊肚菌芳香物相似(Tu et al. 2021),都是食用菌常见的挥发性化合物,如辛-1-烯-3-醇和苯乙醛,代表了食用菌不同的香味。
从六妹羊肚菌子实体的乙醇浸膏中共分离鉴定出14个化合物,包括7个甾体类化合物、3个长链脂肪酸及其酯类化合物和4个芳香酸及其酯类化合物。7个甾体类化合物中有6个为麦角甾类,麦角甾类化合物具有28个碳原子骨架,是真菌中的特征性和代表性化合物(宋明杰和包海鹰 2013)。此外,本研究还从六妹羊肚菌子实体中分离得到两个邻苯二甲酸酯类化合物(化合物2和13)。邻苯二甲酸酯(Pathalic Acid Esters,简称PAEs,别名酞酸酯)为常见的塑料增塑剂,对生物和人类的健康构成一定的威胁。大棚种植作物,如蔬菜、食用菌等可通过根系或者茎叶等途径对PAEs进行吸收累积(林梦茜等 2011),这是以后大棚种植作物需要关注的问题。
参考文献
Use of a free radical method to evaluate antioxidant activity. LWT - Food Science and
Differentiation of aroma characteristics of pine-mushrooms (Tricholoma matsutake Sing.) of different grades using gas chromatography- olfactometry and sensory analysis
DOI:10.1021/jf062702z URL [本文引用: 1]
Votatiles and key odorants in the pileus and stipe of pine-mushroom (Tricholoma matsutake Sing.)
DOI:10.1016/j.foodchem.2007.05.047 URL [本文引用: 1]
Potential aromatic compounds as markers to differentiate between Tuber melanosporum and Tuber indicum truffles
DOI:10.1016/j.foodchem.2013.03.027
PMID:23768334
[本文引用: 1]

The Tuber indicum (Chinese truffle) and Tuber melanosporum (Black truffle) species are morphologically very similar but their aromas are very different. The black truffle aroma is much more intense and complex, and it is consequently appreciated more gastronomically. This work tries to determine whether the differences between the aromatic compounds of both species are sufficiently significant so as to apply them to fraud detection. An olfactometric evaluation (GC-O) of T. indicum was carried out for the first time. Eight important odorants were identified. In order of aromatic significance, these were: 1-octen-3-one and 1-octen-3-ol, followed by two ethyl esters (ethyl isobutyrate and ethyl 2-methylbutyrate), 3-methyl-1-butanol, isopropyl acetate, and finally the two sulfides dimethyldisulfide (DMDS) and dimethylsulfide (DMS). A comparison of this aromatic profile with that of T. melanosporum revealed the following differences: T. indicum stood out for the significant aromatic contribution of 1-octen-3-one and 1-octen-3-ol (with modified frequencies (MF%) of 82% and 69%, respectively), while in the case of T. melanosporum both had modified frequencies of less than 30%. Ethyl isobutyrate, ethyl 2-methylbutyrate and isopropyl acetate were also significantly higher, while DMS and DMDS had low MF (30-40%) compared to T. melanosporum (>70%). The volatile profiles of both species were also studied by means of headspace solid-phase microextraction (HS-SPME-GC-MS). This showed that the family of C8 compounds (3-octanone, octanal, 1-octen-3-one, 3-octanol and 1-octen-3-ol) is present in T. indicum at much higher levels. The presence of 1-octen-3-ol was higher by a factor of about 100, while 1-octen-3-one was detected in T. indicum only (there was no chromatographic signal in T. melanosporum). As well as showing the greatest chromatographic differences, these two compounds were also the most powerful from the aromatic viewpoint in the T. indicum olfactometry. Therefore, either of the two chromatographic methods (GC-O or HS-SPME-GC-MS), together or separately, could be used as a screening technique to distinguish between T. indicum and T. melanosporum and thus avoid possible fraud.Copyright © 2013 Elsevier Ltd. All rights reserved.
Diversity, evolutionary history and cultivation of morels: a review
Chemical constituents from seeds of Helianthus annuus
Effects of different concentrations of plant ash on mycelium growth and sclerotia formation of Morchella sextelata
A novel sterol from Chinese truffles Tuber indicum
DOI:10.1016/S0039-128X(01)00105-2 URL [本文引用: 1]
Determination of mineral contents of wild-grown edible mushrooms
DOI:10.1016/j.foodchem.2008.08.058 URL [本文引用: 1]
Triterpenes and steroids from Armillaria mellea Vahl. ex Fr
DOI:10.1016/j.bse.2007.03.017 URL [本文引用: 1]
Antioxidant activities, metal contents, total phenolics and flavonoids of seven Morchella species
DOI:10.1016/j.fct.2009.06.032 URL [本文引用: 1]
Isolation, characterization and antiproliferative evaluation of constituents from stem extracts of Alafia barteri Olive. Hook. f
DOI:10.1007/s00044-017-2033-4 URL [本文引用: 1]
Chemical constituents from underground part of Astragalus camptodontoides
Larvicidal activity of bis(2-ethylhexyl) benzene- 1,2-dicarboxylate from Sterculia guttata seeds against two mosquito species
Constituents of PG201 (Layla®), a multi-component phytopharmaceutical, with inhibitory activity on LPS-induced nitric oxide and prostaglandin E2 productions in macrophages
DOI:10.1007/s12272-015-0654-z URL [本文引用: 1]
The chemical composition of essential oils and lipophilic extracts of Silphium integrifolium Michx. and Silphium trifoliatum L. rhizomes
Analysis of flavor components in button mushroom soup by HS-SPME-GC-MS and GC-O
Status review of PAEs pollution and its detection method in greenhouse plants
Nutrient analysis of morel in northwest Yunnan province
The gastric protective effects of Morchella conica on ethanol-induced gastric mucosal lesion in rats
Chemical study on the fruiting bodies of Armillariella tabescens
Effects of exopolysaccharide extract from Morchella conica on proliferation and aging of human skin fibroblasts
Dietary linoleic acid human health: focus on cardiovascular and cardiometabolic effects
DOI:S0021-9150(19)31575-8
PMID:31785494
[本文引用: 1]

This narrative review aims to discuss the more relevant evidence on the role of linoleic acid (LA), a n-6 essential fatty acid that constitutes the predominant proportion of dietary polyunsaturated fatty acids (PUFA), in cardiovascular health. Although LA can be metabolized into Arachidonic Acid (AA), a 20 carbon PUFA which is the precursor of eicosanoids, including some with proinflammatory or prothrombotic-vasoconstrictor action, the large majority of experimental and clinical studies have assessed the potential benefit of increasing dietary intake of LA. Overall, data from clinical studies and meta-analyses suggest an association between high dietary intakes or tissue levels of n-6 PUFA, and specifically LA, and the improvement of cardiovascular risk (mainly of the plasma lipid profile), as well as long-term glycaemic control and insulin resistance. Most observational data show that elevated/increased dietary intake or tissue levels of LA is associated with a reduced incidence of cardiovascular diseases (mainly coronary artery diseases) and of new onset metabolic syndrome or type 2 diabetes. The effects of LA (or n-6 PUFA) in other physio-pathological areas are less clear. High quality clinical trials are needed to assess both the actual amplitude and the underlying mechanisms of the health effects related to dietary intake of this essential fatty acid.Copyright © 2019 Elsevier B.V. All rights reserved.
Chemical composition and antimicrobial activity of essential oil from Phytolacca dodecandra collected in Ethiopia
DOI:10.3390/molecules24020342 URL [本文引用: 1]
Antioxidant properties of methanolic extracts from Grifola frondosa, Morchella esculenta and Termitomyces albuminosus mycelia
DOI:10.1016/j.foodchem.2003.10.026 URL [本文引用: 1]
Structural characterization and immunomodulating activities of polysaccharides from a newly collected wild Morchella sextelata
DOI:S0141-8130(18)36907-1
PMID:30771397
[本文引用: 1]

A purified polysaccharide was acquired from a newly collected wild Morchella. The strain identification initially showed that the strain was Morchella sextelata. This study aimed to investigate the structural features and immunomodulating activities of the polysaccharide. Polysaccharide extracted from mycelia was purified by DEAE-cellulose chromatography and Sephadex G-25 size-exclusion chromatography in sequence. The main fraction of polysaccharide (MSP-II) was obtained during purification process. High Performance Liquid Chromatography (HPLC) analysis revealed that MSP-II was composed of Glc, Ara, Gal, Man, Rha, Fuc, GalUA and GluUA in ratio of 34.95:8.7:9.55:4.55:5.0:1.45:12.7:7.65. The structure of MSP-II was furtherly analyzed by FT-IR spectrum and H and C NMR spectroscopy, the results showed that MSP contained β-glycosidic bonds as well as α-glycosidic linkages. In vitro tests proved that MSP-II could not only promote the proliferation and phagocytosis of RAW264.7 cells, but also induce the section of nitric oxide (NO) of macrophages. Consequently, the polysaccharide has a potent immunomodulatory activity by stimulating macrophages and can be considered as a novel potential immunopotentiator in medical and food industries.Copyright © 2019. Published by Elsevier B.V.
Bioactivity and mineral contents of wild-grown edible Morchella conica in the Mediterranean region
DOI:10.1007/s00003-010-0625-8 URL [本文引用: 1]
Antioxidant activity applying an improved ABTS radical cation decolorization assay
A method for the screening of antioxidant activity is reported as a decolorization assay applicable to both lipophilic and hydrophilic antioxidants, including flavonoids, hydroxycinnamates, carotenoids, and plasma antioxidants. The pre-formed radical monocation of 2,2'-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS*+) is generated by oxidation of ABTS with potassium persulfate and is reduced in the presence of such hydrogen-donating antioxidants. The influences of both the concentration of antioxidant and duration of reaction on the inhibition of the radical cation absorption are taken into account when determining the antioxidant activity. This assay clearly improves the original TEAC assay (the ferryl myoglobin/ABTS assay) for the determination of antioxidant activity in a number of ways. First, the chemistry involves the direct generation of the ABTS radical monocation with no involvement of an intermediary radical. Second, it is a decolorization assay; thus the radical cation is pre-formed prior to addition of antioxidant test systems, rather than the generation of the radical taking place continually in the presence of the antioxidant. Hence the results obtained with the improved system may not always be directly comparable with those obtained using the original TEAC assay. Third, it is applicable to both aqueous and lipophilic systems.
Sterol analysis of DMI-resistant and -sensitive strains of Venturia inaequalis
DOI:10.1016/0031-9422(95)00787-3 URL [本文引用: 2]
Research progress of ergot steroid compounds extracted from fungi
Steroids of soft coral Scleronephthya sp. from the south China sea
DOI:10.1007/s10600-012-0245-x URL [本文引用: 1]
Free fatty acid binding pocker in the locked structure of SARS-CoV-2 spike protein
DOI:10.1126/science.abd3255 URL [本文引用: 1]
Chemical composition of aromas and lipophilic extracts from black morel (Morchella importuna) grown in China
DOI:10.1080/12298093.2020.1862473 URL [本文引用: 3]
Antioxidant and antimicrobial activities of Morchella conica Pers
Extraction process optimization, structural characterization and antioxidant activities of polysaccharide from Morchella sextelata
Antihypertensive effects of bioactive extracts from Morchella conica
Resource diversity of Chinese macrofungi: edible, medicinal and poisonous species
DOI:10.1007/s13225-019-00432-7 URL [本文引用: 1]
Physical and chemical properties of polysaccharide isolated from the fruiting bodies of Morchella sextelata and its antioxidant effect. Natural Product Research and Development, 29(7):
Hypolipidemic effect of bioactive extract from Morchella conica
Free radical scavenging properties of conjugated linoleic acids
Conjugated linoleic acids (CLA) were investigated for free radical scavenging properties against the stable 2,2-diphenyl-1-picryhydrazyl radical (DPPH.) by electron spin resonance (ESR) spectrometry and spectrophotometric methods. ESR results demonstrated that CLA directly reacted and quenched free DPPH radicals in benzene, while spectrophotometric analysis showed the radical scavenging capacity of CLA in ethanol. Dose and time effects of CLA-DPPH. reactions were observed in both tests. The ED(50) of CLA was 18 mg/mL under experimental conditions. CLA are much weaker radical scavengers as compared to vitamin E, vitamin C, and BHT. Kinetics of CLA-DPPH. reactions was different to that of linoleic acid (LA)-DPPH. reactions. CLA reacted and quenched DPPH radicals at all tested levels without a lag phase, while LA had a lag phase and showed no radical quenching activity at levels of 5-80 mg/mL in 30 min. These data indicated that CLA can provide immediate protection against free radicals, but LA cannot.
Sesquiterpene glycosides from cotton oil cake
DOI:10.1016/S0031-9422(98)00075-2 URL [本文引用: 1]
Chemical constituents from Saussurea pachyneura
DOI:10.4103/pm.pm_453_19 URL [本文引用: 2]
类芒赤黄芪地下部分化学成分研究