玫烟色虫草Cordyceps fumosorosea旧称玫烟色棒束孢Isaria fumosorosea、玫烟色拟青霉Paecilomyces fumoroseus,是一类重要的虫生真菌,分布广泛(Kepler et al. 2017),可侵染粉虱、蓟马、蚜虫、小绿叶蝉等多种害虫(Mascarin et al. 2013;孟豪 2015),是一种具有应用潜力的重要生防真菌。
温室可提供较稳定的高湿度环境,是应用虫生真菌防治害虫的理想场所,而温室中的短时高温会抑制真菌孢子的萌发,是制约虫生真菌在温室应用的主要瓶颈。因此提高孢子应对短时高温的能力是虫生真菌应用研究的重要内容之一。Magan (2001)认为改变发酵条件、提供适当的环境胁迫可以激发孢子积累内源物质,提高菌株在田间的定殖能力,控制菌株的生理过程以提高菌株的产量和质量。如玉米-玉米油混合基质中不饱和脂肪酸与提高分生孢子耐热性有关(Kim et al. 2010);培养基中添加水杨酸,使得绿僵菌分生孢子的耐热性提高了两倍(Rangel et al. 2012)。孢子的内源物质海藻糖具有渗透调节作用,与真菌繁殖体抵抗外界不良环境的能力有关(Hallsworth & Magan 1996),其机理是海藻糖能维持变性蛋白的半折叠状态,有利于分子伴侣热休克蛋白HSP的加工使其重新折叠而恢复活力(Singer & Lindquist 1998)。
玫烟色虫草的分生孢子和芽生孢子都可以侵染寄主,孢子成功萌发是有效侵染的前提。菌丝体通过细胞酵母式芽殖或者菌丝隔膜间裂殖产生的芽生孢子,是一种具有亲水特性的单壁薄壁酵母样细胞。研究表明芽生孢子比分生孢子能更快地杀死白粉虱和蚜虫(Behle et al. 2006)。此外,液体培养2-3 d的芽生孢子数量与固体培养15 d的分生孢子数量相当(Jaronski & Jackson 2012)。芽生孢子毒力高、对昆虫的感染性强、萌发速度快、产量高,但在环境中的抗逆性较差(紫外辐射和热等)使其应用受到很大限制。若通过发酵技术提高其耐逆性,将有益于芽生孢子的工业化生产和应用。菌丝生长期发酵培养基中营养成分与玫烟色虫草芽生孢子耐热性之间的关系鲜有报道。
鉴于此,本研究测定了碳源种类与浓度对玫烟色虫草IF-1106芽生孢子耐热性的影响,进一步探索了耐热性与胞内海藻糖之间的关系,以期为生产高耐热性的玫烟色虫草芽生孢子提供依据。
1 材料与方法
1.1 供试菌株
玫烟色虫草Cordyceps fumosorosea IF-1106,保藏于中国微生物菌种保藏管理委员会普通微生物中心,保藏编号:CGMCC No.7514。以磁珠菌种保藏管保存于-20 ℃。使用时,将菌株取出活化后接种在PDA培养基上,在25 ℃的霉菌培养箱中培养。
1.2 种子液制备
在产孢良好的PDA平板中加入适量0.1% (V/V)的Tween-80水溶液,用刮板轻刮收集分生孢子,过滤除掉菌丝和培养基残物,得到母液,调整孢子悬浮液浓度为1×107孢子/mL。
1.3 菌株培养
以5%接种量将孢子悬浮液接种至下列液体培养基中,25 ℃、160 r/min培养3 d,每个处理重复3次。基础培养基:MgSO4·7H2O 10 g/L、KCl 10 g/L、KH2PO4 20 g/L、FeSO4·7H2O 2 g/L、蔗糖40 g/L、蛋白胨5 g/L (田晶等 2018)。碳源种类:在基础培养基中,以等质量的葡萄糖、果糖、海藻糖、麦芽糖、可溶性淀粉为碳源代替蔗糖,其他成分不变,以基础培养基为对照。碳源浓度:筛选出最佳碳源为蔗糖,改变蔗糖浓度分别为8、20、40、80、120 g/L。
1.4 高温处理和芽生孢子萌发率的测定
液体发酵3 d后,用4层无菌纱布过滤掉菌丝,将滤液倒入灭菌后的离心管中,4 000 r/min离心30 min,除去上清液,得到芽生孢子。用接种环取一定量的芽生孢子,用萌发液(2%葡萄糖,1%蛋白胨)洗脱,配制孢子悬浮液,使得孢子浓度在显微镜下(放大倍数为10×40)每个视野中约有40个孢子。采用凹玻片悬滴法将芽生孢子悬浮液置于45 ℃处理1、1.5、2、2.5、3 h,后将凹玻片置于25 ℃下培养,观察孢子萌发情况,芽管长度超过孢子直径一半视为萌发,统计孢子萌发率(Stephan & Zimmermann 1998)。每个处理重复3次,每次观察3个视野,取平均值,以未经高温处理的芽生孢子的萌发率为对照。
1.5 液体培养中产孢量和菌丝生物量的测定
液体培养3 d后,用烘干至恒重的擦镜纸过滤菌丝,后用蒸馏水洗涤菌丝并于60 ℃烘箱中烘干,称重,计算菌丝生物量(mg/mL发酵液)。菌液用涡旋仪摇匀,取10 μL滤液与10 μL台盼蓝混匀后用LunaTM自动细胞计数器计数,计算产孢量(孢子/mL发酵液)。
1.6 海藻糖的提取和检测
对Rangel et al. (2008)海藻糖的提取方法进行了修改,具体步骤如下:将芽生孢子冷冻干燥24 h后,称取10 mg干孢子置于含有1 mL超纯水的离心管中,沸水浴5.5 min,后于6 500 r/min离心15 min,取上清液,于4 ℃保存。用离子色谱仪(ICS-3000 Thermo)进行分析。色谱条件:Carbo PACTMPA10 (4 mm×250 mm)阴离子交换柱,Carbo PACTMPA10 (4 mm×50 mm)保护柱,脉冲安培检测器。流动相为5 mmol/L NaOH流速1 mL/min;柱温30 ℃;进样体积20 μL。每个处理重复3次。
1.7 数据处理
采用Origin 2019软件对不同培养基所产芽生孢子的存活指数(Is)-处理时间(t)以存活模型Is=1/(1+exp(a+bt))进行拟合,求出Is为0.5时所对应的处理时间,即计算得到LT50值作为孢子耐热性的评价指标(Ying&Feng 2010)。其中,存活指数(Is)为热处理的芽生孢子与未经热处理的芽生孢子萌发率之比;b为芽生孢子萌发率随热处理时间的下降速率;LT50为Is为0.5时,LT50=-a/b。所有数据采用SPSS 19.0数据处理软件进行显著性分析。
2 结果与分析
2.1 碳源种类对玫烟色虫草IF-1106芽生孢子耐热性和产量的影响
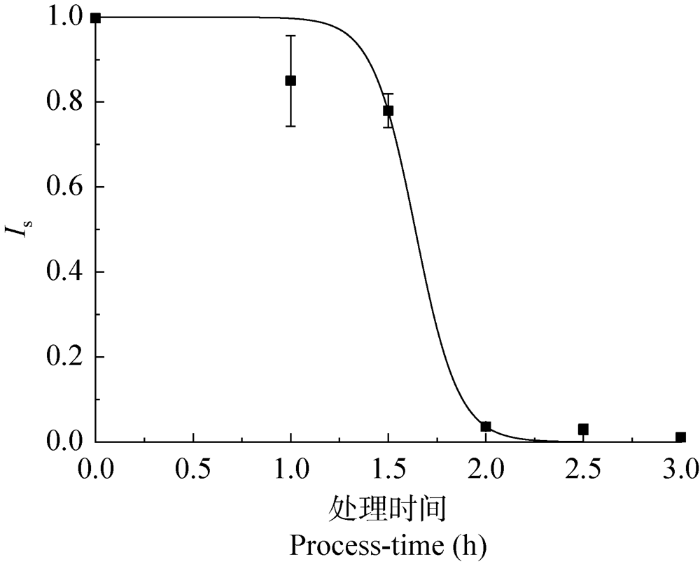
图1
图1
不同处理时间下玫烟色虫草IF-1106芽生孢子的萌发率(45 ℃)
Fig. 1
Germination rate of blastospores of Cordyceps fumosorosea IF-1106 under different treatment times of heat stress (45 °C).
图2
图2
45 ℃处理不同时间玫烟色虫草IF-1106芽生孢子萌发的显微观察 A:无胁迫,B:胁迫1 h,C:胁迫2 h,D:胁迫3 h
Fig. 2
Microscopic observation on the germination of blastospores of Cordyceps fumosorosea IF-1106 at 45 °C under different treatment time. A: No heat stress; B: Under heat stress for 1h; C: Under stress for 2 h; D: Under stress for 3 h.
LT50越大表明芽生孢子的耐热性越好。玫烟色虫草IF-1106在不同碳源培养基所产芽生孢子的耐热性差异显著,蔗糖和果糖培养基所产芽生孢子的耐热性最好,LT50为1.65 h,而葡萄糖培养基所产芽生孢子的耐热性最差,LT50仅为0.94 h (表1)。蔗糖由一分子葡萄糖与一分子果糖脱水缩合而成,在细胞内会分解成葡萄糖和果糖。由此可见,果糖可能是菌株产生耐热性孢子的有效诱导物。据Rangel et al. (2008)报道,在添加果糖的MM培养基上Metarhizium anisopliae产生的分生孢子的耐热性比在添加葡萄糖的MM培养基上产生的分生孢子耐热性高。原因可能是葡萄糖抑制热休克小蛋白基因HSP12和HSP26以及编码的SSA3基因的表达量,因此,在含葡萄糖培养基条件下生长的细胞中,这些基因的表达量较低(Gasch & Werner-Washburne 2002)。但碳源种类对白僵菌孢子耐热性却呈现不同的规律,添加4%葡萄糖增加了白僵菌的耐热性,而添加蔗糖则会降低菌株的耐热性(Kim et al. 2011)。可见,菌株不同,在菌丝培养过程中激发耐热性孢子产生的物质也不同。
表1 不同碳源下所产芽生孢子的存活指数-处理时间拟合方程
Table 1
| 碳源 Carbon source | 存活指数-处理 时间拟合方程 Survival index- treatment time fitting equation | R2 | LT50 (h) |
|---|---|---|---|
| 麦芽糖 Maltose | Is=1/(1+e(-4.50+3.09t)) | 0.958 1 | 1.45±0.08b |
| 可溶性淀粉 Starch soluble | Is=1/(1+e(-6.41+4.55t)) | 0.998 9 | 1.40±0.10b |
| 蔗糖 Sucrose | Is=1/(1+e(-13.51+8.20t)) | 0.979 9 | 1.65±0.27a |
| 果糖 Fructose | Is=1/(1+e(-3.66+2.21t)) | 0.969 7 | 1.65±0.06a |
| 葡萄糖 Glucose | Is=1/(1+e(-3.08+3.25t)) | 0.968 7 | 0.94±0.43c |
| 海藻糖 Trehalose | Is=1/(1+e(-7.54+5.13t)) | 0.979 8 | 1.47±0.10b |
注:单因素方差分析,用最小显著差数法检验平均数,不同字母表示在α=0.05水平上差异显著
Note: One-way analysis of variance, using the least significant difference method to test the average, different letters indicate the level of α=0.05 significant difference.
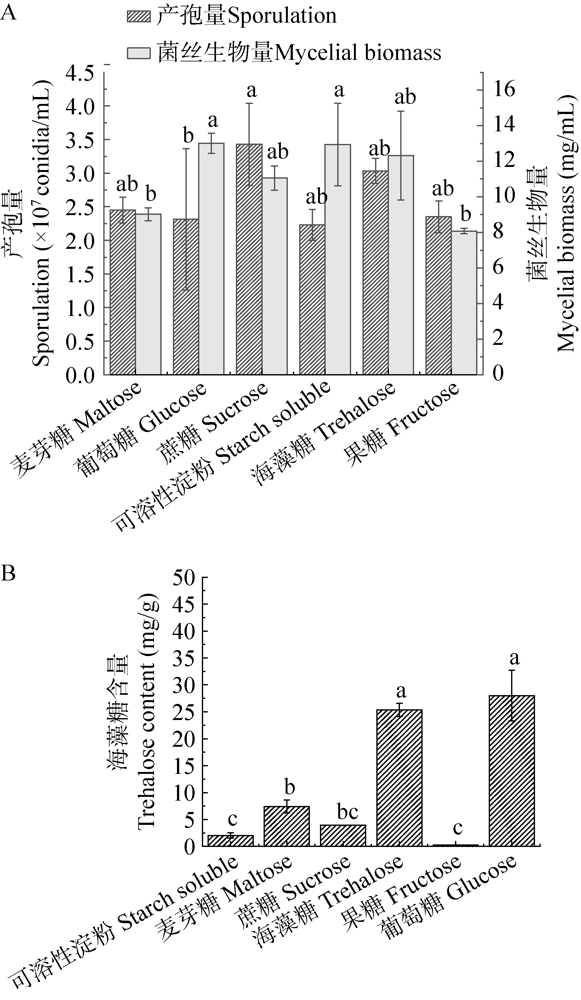
在不同碳源条件下玫烟色虫草芽生孢子的产孢量及菌丝生物量差异显著(图3A)。在蔗糖培养基中产孢量最大,为3.43×107孢子/mL,在葡萄糖、可溶性淀粉、海藻糖培养基中,菌丝生物量均较大,但产孢量却较低。可见,产孢量与菌丝生物量之间为非正向相关关系。葡萄糖、可溶性淀粉、海藻糖可以促进菌丝的生长但却不能诱导大量产孢。
图3
图3
不同碳源条件下芽生孢子的产孢量和菌丝生物量(A)及其胞内海藻糖含量(B) 单因素方差分析,用最小显著差数法检验平均数,不同字母表示在α=0.05水平上差异显著
Fig. 3
Blastospores production and mycelial biomass under different carbon source conditions (A) and intracellular trehalose content (B). One-way analysis of variance, using the least significant difference method to test the average, different letters indicate the level of α=0.05 significant difference.
海藻糖是孢子耐热的关键内源物质。不同碳源培养基所产芽生孢子的胞内海藻糖含量差异显著(P<0.05)。葡萄糖培养基产芽生孢子的胞内海藻糖含量最高,为28.01 mg/g,蔗糖培养基所产孢子胞内海藻糖含量与果糖培养基所产孢子的胞内海藻糖含量之间无显著差异(含量为0.28 mg/g)。可以看出,孢子的耐热性越好其胞内海藻糖含量越低。除此之外,研究发现,在增加外源海藻糖的培养基中所产生的芽生孢子胞内海藻糖含量急剧上升,与Hallsworth & Magan (1994)报道的在生长培养基中加入外源海藻糖可以调控内源海藻糖的含量结果相一致。综上,结合芽生孢子耐热性与产孢量,在液体培养时,优选蔗糖为碳源。
2.2 碳源浓度对玫烟色虫草芽生孢子耐热性和产量的影响
培养基中碳源浓度不仅影响菌丝生长、产孢,还会影响所产孢子的质量,蔗糖含量对芽生孢子的耐热性影响差异显著(表2)。蔗糖含量为40 g/L时,芽生孢子的LT50为1.65 h,含量为120 g/L时,芽生孢子的耐热性最差,LT50为0.84 h。蔗糖浓度过高或过低都不能产生耐热性好的孢子。
表2 不同蔗糖浓度下所产芽生孢子的存活指数-处理时间拟合方程
Table 2
| 蔗糖浓度 Sucrose concentration (g/L) | 存活指数-处理时间拟合方程 Survival index-treatment time fitting equation | R2 | LT50 (h) |
|---|---|---|---|
| 8 | Is =1/(1+e(-7.77+6.80t)) | 0.990 1 | 1.14±0.45b |
| 20 | Is =1/(1+e(-3.61+4.01t)) | 0.985 9 | 0.89±0.18c |
| 40 | Is =1/(1+e(-13.51+8.20t)) | 0.979 9 | 1.65±0.27a |
| 80 | Is =1/(1+e(-5.89+4.81t)) | 0.968 8 | 1.22±0.14b |
| 120 | Is =1/(1+e(-1.80+2.15t)) | 0.890 8 | 0.84±0.04c |
注:单因素方差分析,用最小显著差数法检验平均数,不同字母表示在α=0.05水平上差异显著
Note: One-way analysis of variance, using the least significant difference method to test the average, different letters indicate the level of α=0.05 significant difference.
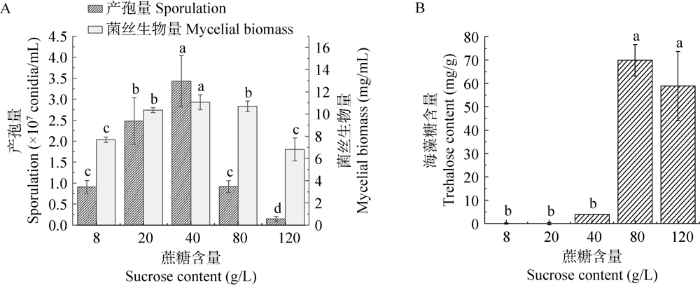
玫烟色虫草芽生孢子在不同蔗糖浓度条件下的产孢量和菌丝生物量见图4A。在不同蔗糖浓度下产孢量及菌丝生物量差异显著。蔗糖浓度对玫烟色虫草生长的影响表现为3种模式,在浓度为40 g/L时,产孢量与菌丝生物量均较大;在浓度20、80 g/L时,菌丝生物量较大,但产孢量却明显降低;在8、120 g/L时,产孢量与菌丝生物量均较低。适宜的碳源浓度是菌丝生长和产孢的必要条件。
图4
图4
蔗糖浓度下芽生孢子的产孢量及菌丝生物量(A)及其胞内海藻糖含量(B) 单因素方差分析用最小显著差数法检验平均数,不同字母表示在α=0.05水平上差异显著
Fig. 4
Blastospore production and mycelial biomass under different sucrose concentration (A) and intracellular trehalose content (B). One-way analysis of variance, using the least significant difference method to test the average, different letters indicate the level of α=0.05 significant difference.
蔗糖浓度对孢子的胞内海藻糖含量影响差异显著。蔗糖浓度为40 g/L时,芽生孢子耐热性最高,此时胞内海藻糖含量较低。蔗糖含量为80 g/L时,孢子的胞内海藻糖含量最高,为69.88 mg/g,其次为120 g/L,可能此时孢子受到高渗透压胁迫而在体内积累了较高浓度的海藻糖(Mascarin et al. 2015);蔗糖含量为8、20 g/L时胞内海藻糖较低,可能是因碳含量过低导致。
3 讨论
温度是衡量昆虫病原真菌耐逆性强弱的一个重要指标,因为环境温度不仅影响孢子的正常萌发,同时也影响着生物制剂的长期贮存和田间稳定性(王定峰等 2010)。本研究选用了田间可能出现的短时间高温(45 ℃、1-3 h)作为玫烟色虫草芽生孢子的热胁迫温度和时长,测定了不同碳源培养条件下该温度胁迫对玫烟色虫草菌株芽生孢子萌发率的影响。结果表明,玫烟色虫草IF-1106菌株芽生孢子在蔗糖为碳源时,孢子的耐热性最好,LT50为1.65 h。可见玫烟色虫草IF-1106芽生孢子具有较强的耐高温能力,是一株非常有应用潜力的生防真菌,这是其可被开发为商业制剂的重要基础。
本研究发现,在玫烟色虫草芽生孢子产生高耐热性的营养条件下,胞内海藻糖含量普遍较低。原因可能是胞内海藻糖积累过多,之后再进行热胁迫,海藻糖的积累继续增加导致积累水平过高可能会抑制变性蛋白的重新激活。海藻糖在热胁迫后可能会发生快速降解,因为它的持续存在会通过分子伴侣干扰变性蛋白的重新折叠(Singer & Lindquist 1998)。而本身胞内海藻糖水平低的芽生孢子,为了应对热胁迫,真菌细胞可能产生转录或者转录后激活的酶,用于海藻糖代谢,从而导致海藻糖积累(Wyatt 1956)。例如,海藻糖合成酶以及中性海藻糖酶都可以通过热胁迫或者其他形式诱导,在热胁迫后胞内海藻糖可能达到一个适宜水平,通过稳定细胞结构和维持蛋白质的天然构象来保护细胞。
昆虫病原真菌对高温的敏感性一直是真菌农药开发的一个有吸引力的研究方向。据报道,在绿僵菌菌丝生产过程中,碳和氮缺乏的营养胁迫下产生的分生孢子海藻糖的积累量显著增加,这种胞质对应激条件的反应至关重要(Elbein et al. 2003;Ruijter et al. 2003)。在这些条件下,细胞对45 ℃的耐受性比在富培养基PDAY (马铃薯葡萄糖琼脂加酵母浸粉)上产生的细胞高2倍(Rangel et al. 2008)。然而,渗透胁迫下产生的分生孢子的耐热性与营养胁迫下产生的孢子相似,但是它们的海藻糖含量很低,说明这些化合物的积累并不是绿僵菌抵御不利环境的唯一机制。这些研究表明,海藻糖的积累和代谢在菌株耐热性方面扮演着重要的角色,但是这个因素不是唯一控制耐热性的原因,因为其他内源物质的积累和代谢也可能参与应激反应,通过上调或下调海藻糖的浓度,海藻糖酶的水平也可能影响其他内源物质的积累水平和整个代谢过程,因此,需要进一步的研究以了解更多提高菌株耐热性的作用机制。
本文主要研究了热胁迫之前营养成分对玫烟色虫草芽生孢子胞内海藻糖的水平变化,但未对胁迫之后胞内海藻糖含量变化进行研究,而热胁迫之后胞内海藻糖的水平变化来解释耐热性与内源物之间的关系将是下一步研究的重点。总之,营养条件会影响细胞的耐热性和细胞成分,因此可以通过液体发酵技术的创新生产耐热的芽生孢子。目前对真菌繁殖体内源物含量的调控研究较少,本研究结果将有助于通过代谢调控手段来增强生防真菌的耐热性能。
参考文献
Pathogenicity of blastospores and conidia of Paecilomyces fumosoroseus against larvae of the mexican bean beetle, Epilachna varivestis Mulsant
New insights on trehalose: a multifunctional molecule
Trehalose is a nonreducing disaccharide in which the two glucose units are linked in an alpha,alpha-1,1-glycosidic linkage. This sugar is present in a wide variety of organisms, including bacteria, yeast, fungi, insects, invertebrates, and lower and higher plants, where it may serve as a source of energy and carbon. In yeast and plants, it may also serve as a signaling molecule to direct or control certain metabolic pathways or even to affect growth. In addition, it has been shown that trehalose can protect proteins and cellular membranes from inactivation or denaturation caused by a variety of stress conditions, including desiccation, dehydration, heat, cold, and oxidation. Finally, in mycobacteria and corynebacteria, trehalose is an integral component of various glycolipids that are important cell wall structures. There are now at least three different pathways described for the biosynthesis of trehalose. The best known and most widely distributed pathway involves the transfer of glucose from UDP-glucose (or GDP-glucose in some cases) to glucose 6-phosphate to form trehalose-6-phosphate and UDP. This reaction is catalyzed by the trehalose-P synthase (TPS here, or OtsA in Escherichia coli ). Organisms that use this pathway usually also have a trehalose-P phosphatase (TPP here, or OtsB in E. coli) that converts the trehalose-P to free trehalose. A second pathway that has been reported in a few unusual bacteria involves the intramolecular rearrangement of maltose (glucosyl-alpha1,4-glucopyranoside) to convert the 1,4-linkage to the 1,1-bond of trehalose. This reaction is catalyzed by the enzyme called trehalose synthase and gives rise to free trehalose as the initial product. A third pathway involves several different enzymes, the first of which rearranges the glucose at the reducing end of a glycogen chain to convert the alpha1,4-linkage to an alpha,alpha1,1-bond. A second enzyme then releases the trehalose disaccharide from the reducing end of the glycogen molecule. Finally, in mushrooms there is a trehalose phosphorylase that catalyzes the phosphorolysis of trehalose to produce glucose-1-phosphate and glucose. This reaction is reversible in vitro and could theoretically give rise to trehalose from glucose-1-P and glucose. Another important enzyme in trehalose metabolism is trehalase (T), which may be involved in energy metabolism and also have a regulatory role in controlling the levels of trehalose in cells. This enzyme may be important in lowering trehalose concentrations once the stress is alleviated. Recent studies in yeast indicate that the enzymes involved in trehalose synthesis (TPS, TPP) exist together in a complex that is highly regulated at the activity level as well as at the genetic level.
The genomics of yeast responses to environmental stress and starvation
Culture age, temperature and pH affect the polyol and trehalose contents of fungal propagules
Effect of carbohydrate type and concentration on polyhydroxy alcohol and trehalose content of conidia of three entomopathogenic fungi
DOI:10.1099/00221287-140-10-2705 URL [本文引用: 1]
Mass production of entomopathogenic Hypocreales
A phylogenetically-based nomenclature for Cordycipitaceae (Hypocreales)
DOI:10.5598/imafungus.2017.08.02.08
PMID:29242779
[本文引用: 1]

The ending of dual nomenclatural systems for pleomorphic fungi in 2011 requires the reconciliation of competing names, ideally linked through culture based or molecular methods. The phylogenetic systematics of and its many genera have received extensive study in the last two decades, however resolution of competing names in has not yet been addressed. Here we present a molecular phylogenetic investigation of that enables identification of competing names in this family, and provides the basis upon which these names can be maintained or suppressed. The taxonomy presented here seeks to harmonize competing names by principles of priority, recognition of monophyletic groups, and the practical usage of affected taxa. In total, we propose maintaining nine generic names, and and the rejection of eight generic names,,,, and. Two new generic names, and, and a new species,, are described. New combinations are also proposed in the genera and
Production of thermotolerant entomopathogenic Isaria fumosorosea SFP-198 conidia in corn-corn oil mixture
Production of thermotolerant entomopathogenic fungal conidia on millet grain
Physiological approaches to improving the ecological fitness of fungal biocontrol agents in fungi as biocontrol agents
Glucose concentration alters dissolved oxygen levels in liquid cultures of Beauveria bassiana and affects formation and bioefficacy of blastospores
The virulence of entomopathogenic fungi against Bemisia tabaci biotype B (hemiptera: aleyrodidae) and their conidial production using solid substrate fermentation
DOI:10.1016/j.biocontrol.2013.05.001 URL [本文引用: 1]
Insecticidal spectrum measurement of Isaria fumosorosea IF-1106 and comparison with pathogenicity of Beauveria bassiana
Evaluating physical and nutritional stress during mycelial growth as inducers of tolerance to heat and UV-B radiation in Metarhizium anisopliae conidia
DOI:10.1016/j.mycres.2008.04.013 URL [本文引用: 2]
Culture of Metarhizium robertsii on salicylicacid supplemented medium induces increased conidial thermotolerance
DOI:10.1016/j.funbio.2012.01.003 URL [本文引用: 1]
Mannitol is required for stress tolerance in Aspergillus niger conidiospores
DOI:10.1128/EC.2.4.690-698.2003
PMID:12912888
[本文引用: 1]

D-Mannitol is the predominant carbon compound in conidiospores of the filamentous fungus Aspergillus niger and makes up 10 to 15% of the dry weight. A number of physiological functions have been ascribed to mannitol, including serving as a reserve carbon source, as an antioxidant, and to store reducing power. In this study, we cloned and characterized the A. niger mpdA gene, which encodes mannitol 1-phosphate dehydrogenase (MPD), the first enzyme in the mannitol biosynthesis pathway. The mpdA promoter contains putative binding sites for the development-specific transcription factors BRLA and ABAA. Furthermore, increased expression of mpdA in sporulating mycelium suggests that mannitol biosynthesis is, to a certain extent, developmentally regulated in A. niger. Inactivation of mpdA abolished mannitol biosynthesis in growing mycelium and reduced the mannitol level in conidiospores to 30% that in the wild type, indicating that MPD and mannitol 1-phosphate phosphatase form the major metabolic pathway for mannitol biosynthesis in A. niger. The viability of spores after prolonged storage and germination kinetics were normal in an mpdA null mutant, indicating that mannitol does not play an essential role as a reserve carbon source in A. niger conidia. However, conidiospores of a DeltampdA strain were extremely sensitive to a variety of stress conditions, including high temperature, oxidative stress and, to a lesser extent, freezing and lyophilization. Since mannitol supplied in the medium during sporulation repaired this deficiency, mannitol appears to be essential for the protection of A. niger spores against cell damage under these stress conditions.
Multiple effects of trehalose on protein folding in vitro and in vivo
The disaccharide trehalose is produced in large quantities by diverse organisms during a variety of stresses. Trehalose prevents proteins from denaturing at high temperatures in vitro, but its function in stress tolerance in vivo is controversial. We report that trehalose stabilizes proteins in yeast cells during heat shock. Surprisingly, trehalose also suppresses the aggregation of denatured proteins, maintaining them in a partially-folded state from which they can be activated by molecular chaperones. The continued presence of trehalose, however, interferes with refolding, suggesting why it is rapidly hydrolyzed following heat shock. These findings reconcile conflicting reports on the role of trehalose in stress tolerance, provide a novel tool for accessing protein folding intermediates, and define new parameters for modulating stress tolerance and protein aggregation.
Development of a spray-drying technique for submerged spores of entomopathogenic fungi
Optimization technology of liquid fermentation of Isaria fumosorosea IF-1106 strain: China, CN108342329A
Biochemical and molecular bases of stress-tolerance of entomogenous fungi and their application
The chemistry of insect hemolymph
Relationship between thermotolerance and hydrophobin-like proteins in aerial conidia of Beauveria bassiana and Paecilomyces fumosoroseus as fungal biocontrol agents
DOI:10.1111/jam.2004.97.issue-2 URL [本文引用: 1]