萜类化合物种类丰富,是天然产物的最大家族,到目前为止,已经发现了95 000多种结构不同的萜类化合物(Quin et al. 2014;Christianson 2017)。这类化合物结构具有(C5H8)n通式,分子骨架是以异戊二烯单元为基本结构单元的化合物及其衍生物。它们普遍存在于自然界中,是构成植物香精、树脂和色素等次级代谢产物的主要成分(李传旺等 2021)。萜类化合物生物合成的共同前体是异戊二烯焦磷酸(isopentenyl diphosphate, IPP)和二甲基烯丙基焦磷酸(dimethylallyl pyrophosphate, DMAPP),根据IPP生物合成来源和机制的不同可以将萜类化合物的生物合成分为两条不同的途径,分别是甲羟戊酸途径(MVA途径)和甲基赤藓糖磷酸途径(MEP途径) (Kuzuyama 2002)。萜类化合物按照碳原子的数目可以分为单萜(C10)、倍半萜(C15)、二萜(C20)、三萜(C30)和多萜等。由于多数萜类化合物分子中具有不同的碳环数,因此又可以分为链萜、单环萜、双环萜和三环萜等(刘莉和胡昌华 2010)。由此可见,萜类化合物从简单的线型碳氢链结构到复杂的环化结构充分体现了其结构多样性,这种结构多样性在一定程度上决定了它们生物活性的多样性(Gershenzon & Dudareva 2007)。
二萜类化合物作为萜类化合物的代表之一,广泛存在于植物和真菌中,是一类具有重要价值的天然产物,并且以其广泛的生物活性成为国内外的研究热点。根据目前的文献记载显示,真菌中发现的二萜都是由MVA途径合成而来。IPP在Ⅰ型或者Ⅱ型IPP异构酶的作用下异构化形成DMAPP (刘莉和胡昌华 2010),然后DMAPP在香叶基香叶基焦磷酸合成酶(geranylgeranyl diphosphate synthase, GGPPS)的作用下再与3分子的IPP缩合形成香叶基香叶基焦磷酸(geranylgeranyl diphosphate, GGPP) (Ohnuma et al. 1998)。大部分功能性二萜是由非环状的GGPP在二萜环化酶的作用下生成二萜母核骨架,然后再经过进一步的加工修饰如氧化、还原、甲基化和糖基化等得到最终产物(刘莉和胡昌华 2010)。二萜类化合物结构的复杂性和多样性,使其具备了多种不同的生物活性,因此,这类化合物被广泛用作临床药物,具有非常高的应用和经济价值,从而受到越来越多的关注(益萱等2019)。例如,从大型真菌中分离出的一些鸟巢烷型二萜化合物(cyathane)已被证明可以促进某些神经因子生长或合成,是治疗某些神经退行性疾病如阿尔茨海默病的先导化合物(汪锴等 2015)。
本文总结了大型担子菌发现的6大类典型二萜化合物的化学结构、生物活性以及生物合成途径,将为担子菌中二萜类化合物生物活性和生物合成途径解析提供参考,同时对其生物活性挖掘利用提供理论依据。
1 大型担子菌中6种二萜类化合物基本骨架的概述
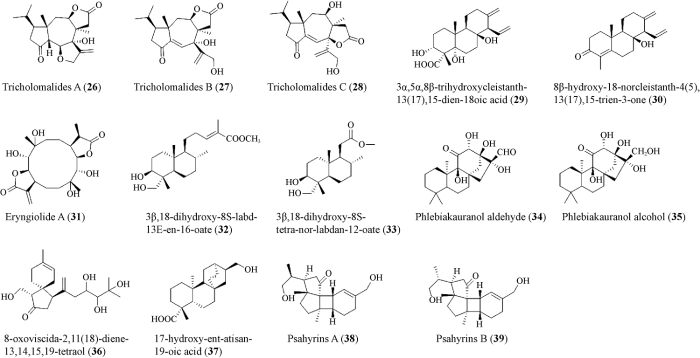
根据目前从大型担子菌中发现的二萜类化合物结构特点可将其分为6大类,分别为鸟巢烷(cyathanes)型、截短侧耳素(pleuromutilins)型、guanacastanes型、海松烷(pimaranes)型、松香烷(abietanes)型和毛皮伞烷(crinipellins)型(图1)。
图1
图1
大型担子菌中二萜化合物6种类型及化合物
Fig. 1
Six types and compounds of diterpenoids in macro-basidiomycetes.
1.1 鸟巢烷(cyathanes)型二萜
Cyathanes型二萜,即鸟巢烷型二萜,是担子菌二萜类化合物中数量最多的类型之一(Zhang & Feng 2022)。根据近十年来的文献报道已经有近百种该类型二萜被发现。鸟巢烷型二萜主要从Cyathus属、Hericium属和Sarcodon属以及3个特殊物种Phellodon niger、Laxitextum incrustatum和Strobilurus tenacellus中分离得到(Chen & Liu 2017)。此类化合物最初于1971年从看起来像鸟巢的Cyathus属中分离出来,并以此命名(Johri & Brodie 1971)。例如,从胡可黑蛋巢菌Cyathus hookeri中的Martin液体培养物中分离出一个cyathanes型二萜蛋巢菌素(cyathinⅠ) (1),该化合物对脂多糖激活的巨噬细胞中的一氧化氮合成具有抑制作用,IC50为15.5 μmol/L (Xu et al. 2013)。该类化合物具有5/6/7三元环杂合基本骨架,且表现出多种生物活性(Yin et al. 2019),包括刺激神经突起的生长和神经元营养的双功能、抗炎、抗菌及抗肿瘤等(张成宸 2016;彭勤颖等 2021)。
Kawagishi et al. (1994)从猴头菌Hericium erinaceum液体发酵菌丝体中首次分离并鉴定了含有鸟巢烷基本骨架和木糖基团的二萜化合物erinacine A-C (2-4)。Tzeng et al. (2016)证实了化合物2能够改善阿尔茨海默病的病理症状,表现为可减轻脑部斑块沉积、提高大脑皮层胰岛素降解酶的水平、增加神经生长因子(nerve growth factor, NGF)与NGF前体的比值以及促进海马神经生长。Bailly & Gao (2020)在该类化合物的活性作用机制方面做了系统性的论述,化合物2可以通过干扰TrkA-NGF受体-配体复合物来调节NGF的刺激效应的能力,从而发挥神经保护作用;同时诱导神经细胞内各种过氧化物酶的产生,减少毒素激发的细胞凋亡从而起到保护神经的作用。正是由于鸟巢烷二萜的生物活性,此类化合物有望被开发为神经保护剂,应用于包括阿尔茨海默病、帕金森病等神经退行性疾病(张成宸 2016)。
1.2 截短侧耳素(pleuromutilins)型二萜
Kavanagh & Robbins (1951)首次在2种担子菌中(Pleurotus passeckerianus和亚脐菇杯状斜盖伞Clitopilus scyphoides)分离了截短侧耳素(pleuromutilins) (5)。该化合物的结构特点是具有5/6/8三环骨架组成。化合物5通过与50S核糖体亚基的相互作用抑制原核蛋白合成而发挥其抗菌活性(Hodgin & Högenauer 1974;Hgenauer 1975;Poulsen et al. 2001)。另外,化合物5对青霉素和链霉素耐药的葡萄球菌和支原体都显示出更强的抑制活性。为了提高抗菌活性,人们合成了许多化合物5的衍生物,到目前为止,已经批准了4种化合物5的衍生物用于感染性疾病,包括作为兽药的tiamulin (6)和valnemulin (7)、作为治疗疱疹病毒的抗生素retapamulin (8)以及用于治疗社区获得性细菌性肺炎(CABP)的lefamulin (9) (Dornhelm & Högenauer 1978;Poulsen et al. 2001;Yan et al. 2006;Paukner et al. 2013)。此外,Guo et al. (2022)从Omphalina mutila固体培养物中分离得到一系列化合物5的衍生物,其中,化合物pleuromutilins C (10)对金黄色葡萄球菌和粪肠球菌有较强的抑制作用。
1.3 guanacastanes型二萜
Guanacastanes型二萜的特征是含有5/7/6环融合结构,该类型化合物在大型真菌的次生代谢产物中比较少见,主要来自于Coprinus属(Kinghorn et al. 2017)。该类化合物对癌细胞具有较强的杀伤作用(Ou et al. 2012)。其中从C. radians中分离出的化合物radianspene C (11)对人乳腺癌细胞具有生长抑制活性,IC50值为0.91 μmol/L。从C. plicatilis中分离出的化合物plicatilisin A (12)对HepG2、HeLa、MDA-MB-231、BGC-823和HCT 116人癌细胞具有细胞毒作用,IC50值在1.2-6.0 μmol/L之间(Liu et al. 2012)。2014年从担子菌Psathyrella candollana的培养物中分离得到化合物guanacastane R (13),化合物13对人和小鼠的11β-HSD1同工酶均有抑制活性,IC50值分别为6.2和13.9 μmol/L (Yin et al. 2014)。由于guanacastane复杂的多环结构和良好的生物活性,其总合成也引起了人们的关注,Gampe & Carreira (2011)实现了guanacastepene N (14)和guanacastepene O (15)的全合成研究。这将有助于进一步研究此类二萜的生物性质。
1.4 海松烷(pimaranes)型二萜
Pimaranes型二萜的特征是含有6/6/6融合的碳环体系(Reveglia et al. 2018)。该类型二萜大多数在植物和子囊菌中发现,而在担子菌中被报道的pimaranes型二萜并不多。
1.5 松香烷(abietanes)型二萜
Abietanes类二萜属于三环二萜类化合物,大多数具有异构体和旋光性,且骨架多变,结构复杂。Abietanes类二萜中的A、B、C 3个环常带有不同的官能团,常以C环是否氧化脱氢等不同形式的变化使结构丰富多样(张义等 2019)。Abietanes型二萜主要作为植物次级代谢产物存在。具有一定的抗肿瘤、抗炎和抗菌等药理作用。Wang et al. (2006)和Jang & Yang (2011)分别从Phellinus igniarius和Phellinus pini中分离出2个此类型二萜,12-hydroxy-7-oxo-5,8,11, 13-tetraene-18,6-abitanolide (19)和dehydroabietic acid (20),其中20能够轻微抑制NO的生成,IC50值为98.9 μmol/L。Hypoxylon rickii的次生代谢产物rickitin A (21)对金黄色葡萄球菌DSM 346表现出较弱的抗菌活性,MIC值为33.3 μg/cm3。对KB3.1宫颈癌细胞株和L929小鼠成纤维细胞进行细胞毒作用,IC50值分别为18.0和23.0 μg/cm3 (Kuhnert et al. 2015)。Liu et al. (2014)从桦褐孔菌Inonotus obliquus中分离出一个该类型化合物,即inonotusic acid (22),体外实验表明,化合物22对d-氨基半乳糖诱导的WB-F344细胞损伤有明显的保护作用,其抑制率为34.4%-81.2%。
1.6 毛皮伞烷(crinipellins)型二萜
Crinipellins是一类具有四奎烷骨架的二萜化合物,这些化合物由大型担子菌Crinipellis产生。直到1985年,crinipellins的结构才被确定(Chen & Liu 2017)。2017年,从Crinipellis物种的液体培养物中分离出了4种crinipellins型二萜,其中crinipellin E (23)具有抗炎作用。生物实验表明,该化合物对LPS/IFN-γ诱导的瞬时转染人MonoMac6细胞CXCL10启动子活性具有剂量依赖性,IC50值为15 μmol/L (Rohr et al. 2017)。Han et al. (2018)从C. rhizomaticola的培养滤液中分离得到了2个crinipellins型二萜crinipellin A (24)和crinipellin I (25),化合物24对金黄色葡萄球菌、稻瘟病菌、灰霉病菌和马铃薯晚疫病菌具有广泛的体外抗真菌活性(MICs分别为1、8、31和31 µg/mL)。
1.7 其他类型二萜
最近几年在大型担子菌中还产生了其他类型的二萜类化合物,扩大了已知大型担子菌产生萜类化合物的多样性(Chen & Liu 2017) (图2)。Tsukamoto et al. (2003)从口蘑属Tricholoma sp.子实体的甲醇提取物中分离得到3个新的二萜tricholomalides A-C (26-28),并根据其光谱数据对其结构进行了鉴定。在100 μmol/L浓度下,26-28显著诱导大鼠肾上腺嗜铬细胞瘤细胞(PC-12)的突起生长。Zhou et al. (2009)对合生地花菌Albatrellus confluens进行培养,得到了2种新型二萜,分别是3α,5α,8β-trihydroxycleistanth- 13(17),15-dien-18oic acid (29)和8β-hydroxy-18- norcleistanth-4(5),13(17),15-trien-3-one (30)。Wang et al. (2012)从食用菌Pleurotus eryngii的固体培养物中分离出一个具有新型骨架的二萜eryngiolide A (31),它是由1个环十二烷核与2个γ-内酯单元融合而成的。在体外实验中,它对2种人类癌症细胞系(Hela和HepG2)显示出中等的细胞毒性。在生物遗传学上,该化合物可能是第一个未通过GGPP单元合成的二萜,这为自然界中二萜的生物合成开辟了一条全新的途径。Lee et al. (2015)和Kinghorn et al. (2017)从粉红枝瑚菌Ramaria formosa的子实体中分离出2种半日花烷型(labdane)二萜(32, 33),生物活性实验表明,这2种化合物对人类中性粒细胞弹性蛋白酶均有一定抑制作用。其他类型的二萜类化合物,如kauranes型(34, 35)、viscidane型(36)、atisane型(37),代表从大型担子菌中分离出来的一组混合的二萜类化合物(Anke et al. 1987;Arnone et al. 2005;Ying et al. 2014;Zhang et al. 2015)。Liu et al. (2020)从黄盖小脆柄菇Psathyrella candolleana的培养物中鉴定了2个具有新型骨架的四环二萜化合物Psahyrins A (38)和Psahyrins B (39),它们具有新颖的5/5/4/6融合环体系,实验表明,化合物38和39抑制了葡萄球菌和肠道沙门氏菌的生长。
图2
图2
大型担子菌中其他类型二萜化合物
Fig. 2
Other types of diterpenoids in macro-basidiomycetes.
2 大型担子菌中二萜化合物的生物合成研究进展
2.1 Pleuromutilin的生物合成途径
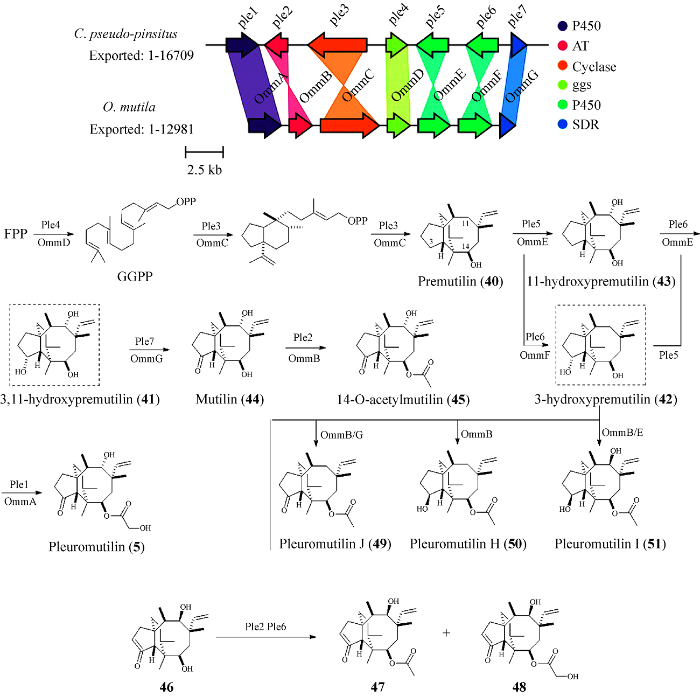
Pleuromutilin (5)是具有良好前景的用于人类疾病治疗的抗生素(Poulsen et al. 2001),但随着对新抗生素需求的不断增长,专门用于人类化合物5的衍生物开发急剧增加(Bailey et al. 2016)。Bailey et al. (2016)在C. passeckerianus中发现了一种二萜合成酶(Pl-cyc)。它是负责催化化合物5的关键二萜合成酶。该课题组通过RT-PCR、Northern分析和基因沉默的方法确定了合成化合物5的基因簇。由于在天然宿主中对该基因簇操作没能实现该化合物产量的增加,因此在米曲霉Aspergillus oryzae中重建了化合物5的总生物合成。从C. passeckerianus中克隆所需7个基因的cDNA序列,并将其在米曲霉中异源表达,重组菌株化合物5的最高产量可达84.24 mg/L,与野生型菌株化合物5的产量8 mg/L相比,增加了11倍。
Yamane et al. (2017)利用GGPP合成酶对C. pseudopinsitus基因组的测序数据进行BLAST搜索,搜索出了一条簇(Ple)具有GGPP合成酶、萜烯合成酶、细胞色素P450、短链脱氢酶和酰基转移酶(图3)。该基因簇结构与Bailey et al. (2016)的报道非常相似。之后该课题组通过米曲霉异源表达和体外酶反应实验提出了参与化合物5生物合成的所有生物合成酶的功能特征,并阐明了生物合成路径(图3)。法尼基焦磷酸(FPP)经Ple4 (GGPP合酶)催化形成GGPP,Ple3 (Pl-cyc)将GGPP转化为premutilin (40),化合物40在Ple5和Ple6 (P450)的作用下,分别在五元环的C-3位和八元环的C-11位发生羟基化生成化合物3,11-hydroxypremutilin (41),当Ple6单独催化化合物40时,则只在五元环的C-3位发生羟基化产生化合物3-hydroxypremutilin (42),同时也证明了化合物42通过Ple5的催化作用可以产生41,化合物40经Ple5催化,在八元环C-11发生羟基化产生11-hydroxypremutilin (43),这些结果揭示了从化合物40到化合物41的两条氧化途径参与了生物合成。化合物40在Ple5、Ple6和Ple7 (短链脱氢酶)的共同作用下生成mutilin (44),化合物44在Ple2 (乙酰转移酶)的催化下生成化合物14-O-acetylmutilin (45),随后Ple1 (氧化还原酶)催化45的乙酰基侧链的α位置上的羟基化从而生成最终化合物5。同时,通过体外酶反应和突变分析实验表明,二萜合酶Ple3催化GGPP到具有特征性5/6/8-三环碳骨架的化合物40的2轮环化。
图3
图3
Pleuromutilin的基因簇、生物合成途径及衍生物的生物转化
Fig. 3
Gene cluster, biosynthetic pathway of pleuromutilin and biotransformation of pleuromutilin derivatives.
Alberti et al. (2017)通过在米曲霉中的异源表达,对参与化合物5生物合成的步骤进行了功能表征。进一步建立了米曲霉作为化学修饰的化合物5衍生物生物转化的平台,并将其用于合成比化合物5活性更强的衍生物。在化合物46存在下培养含有乙酰转移酶和P450的米曲霉菌株(Ple2和Ple6基因),证实了化合物46可以转化为化合物47和化合物48 (图3)。随后通过平板生物测定法研究了化合物5、47和48对枯草杆菌的抗菌活性。实验表明,化合物47与5的抗菌活性相当,化合物47对枯草杆菌生长的抑制区为(12.7±0.6) mm,化合物5为(12.8±0.8) mm。化合物48的抗菌活性要强于化合物47和5,化合物48对枯草杆菌生长的抑制率为(15.7±1.8) mm。这为获得有潜力的半合成衍生物开辟了新的途径。
Guo et al. (2022)鉴定了Omphalina mutila中化合物5的生物合成基因簇(omm基因簇) (图3),并在酿酒酵母中进行了表达。二萜合成酶(ommC)导入酿酒酵母后产生12.2 mg/L的化合物40,然后,将2个细胞色素P450酶(ommE/F)整合到重组菌株中获得了化合物42和41,随后在短链脱氢酶/还原酶(ommG)的作用下生成化合物44,化合物44在酰基转移酶(ommB)的催化下生成化合物45,在细胞色素P450酶(ommA)的催化下最终生成化合物5。随后通过提高ommA的拷贝数使化合物5的效价比亲本菌株提高了7.3倍(从5.8 mg/L增加到42.4 mg/L)。并且通过在酿酒酵母中与不同修饰酶共表达二萜环化酶,获得了3种新的“非天然”pleuromutilins (49-51),其中pleuromutilin I (51)对金黄色葡萄球菌和粪肠球菌具有较强的抗菌活性。
2.2 猴头菌素的生物合成途径
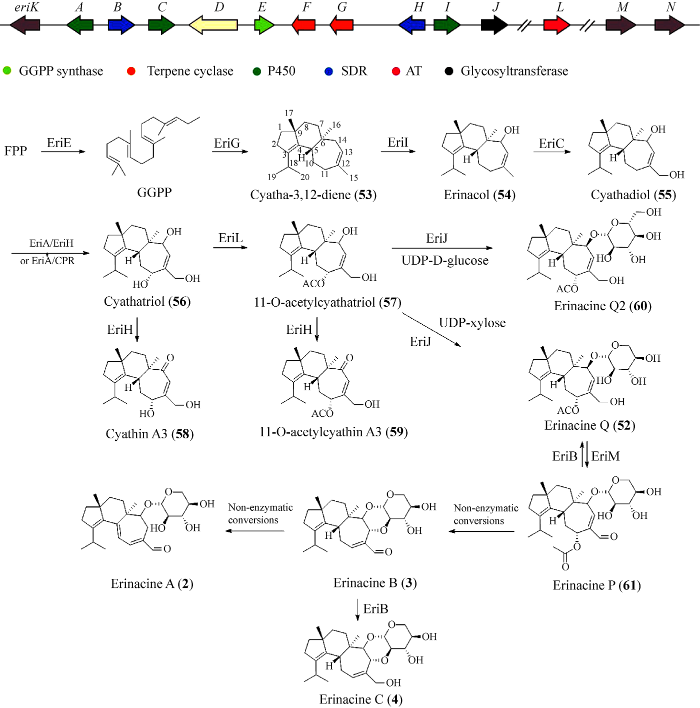
Yang et al. (2017)在猴头菌的基因组中发现一条猴头菌素的生物合成基因簇,该基因簇由EriE (GGPP合成酶)、EriG (ubiA型二萜环化酶)、EriACI (P450酶)、EriJ (糖基转移酶)和EriBH (短链脱氢酶)组成,利用体外酶反应实验鉴定了EriG是合成cyahtine基本骨架的二萜合成酶(图4)。由于该基因簇缺少乙酰转移酶基因,而乙酰转移酶基因可能是生物合成猴头菌素所必需的基因(Kenmoku et al. 2004),因此Liu et al. (2019)重新检查了猴头菌基因组来寻找其他相关基因,以参与化合物5生物合成的Ple2 (乙酰转移酶)为查询序列寻找乙酰转移酶(Yamane et al. 2017),鉴定出同源基因eriL。随后在米曲霉中重组了erinacine生物合成基因簇并阐明了erinacine Q (52)的生物合成路径(Liu et al. 2019) (图4)。FPP在EriE的催化下形成GGPP,GGPP在EriG的作用下转化为(-)-cyatha-3,12-diene (53),化合物53经过EriI的催化在七元环烯丙基C-14处羟基化产生erinacol (54),随后化合物54的七元环C-15处被EriC催化发生羟基化随即产生cyathadiol (55)。EriA需要在EriH或CPR (细胞色素P450还原酶)的共同参与下起到催化作用,催化化合物55的C-11处发生羟基化产生化合物cyathatriol (56),化合物56又在EriL的催化下生成11-O-acetylcyathatriol (57),EriH进一步催化化合物56和57分别产生cyathin A3 (58)和11-O-acetylcyathin A3 (59),糖基转移酶EriJ催化化合物56与糖基供体UDP-木糖发生糖基化反应产生化合物52,当以UDP-D-葡萄糖为糖基供体时可产生化合物erinacine Q2 (60)。化合物60的转化效率比化合物52低8倍,说明EriJ的天然糖供体底物为木糖基(C5H9O5)而不是葡萄糖基(C6H11O6)。
图4
图4
Erinacines基因簇及生物合成途径
Fig. 4
Gene clusters and biosynthetic pathways of erinacines.
Ma et al. (2021)在上述研究成果的基础上鉴定了一个非聚集的FAD依赖性氧化酶EriM和一个依赖于NADP (H)的还原酶EriB的功能,同时,还证明了erinacines A-C (2-4)生物合成中烯丙醛引发的非酶促反应。该课题组鉴定了在C-15处负责醛缩的酶。在与eri簇相似的梅里克式菌Rickenella mellea的rim簇中发现了2个FAD依赖性氧化酶基因(RimF和RimL),以这2个基因为查询序列,从猴头菌中鉴定出同源基因EriM (RimL相似度65.6%)。随后通过酵母异源表达以确定EriM的功能,结果表明,重组菌株产生了代谢产物erinacine P (61),接下来,该课题组又证明EriB能够将化合物61和3分别转化为52和4。根据Kenmoku et al. (2000)报道的化合物61通过Michael加成消除将化合物3转化为化合物2,提出了化合物2形成的非酶促反应。随后通过底物喂养化合物61和体外培养实验后经 HPLC分析显示,化合物61能够转化为化合物2 (产率29%),证明了由化合物61生物合成化合物2、3的过程中存在FAD酶驱动的自发Michael加成消除反应。
3 大型担子菌中Ⅰ型二萜合成酶
图5
图5
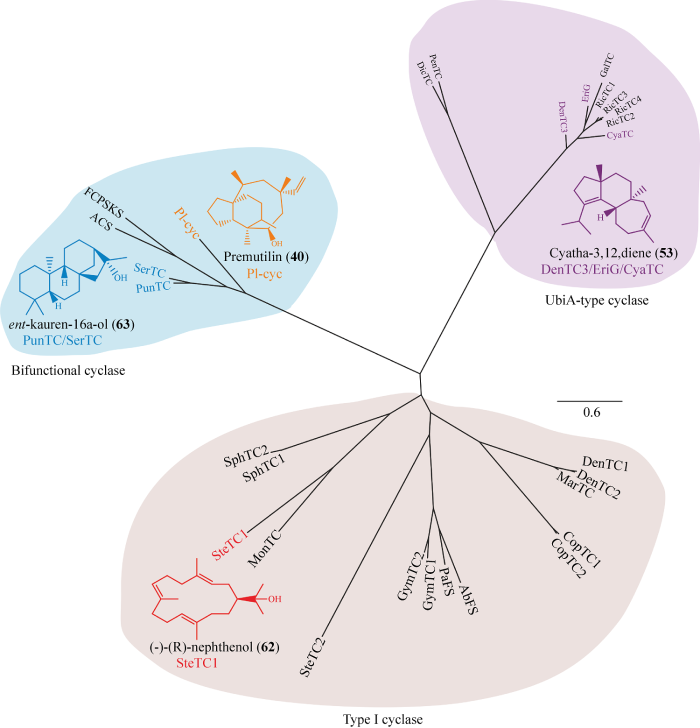
大型担子菌中二萜合成酶的系统发育分析及相应的环化产物
Fig. 5
Phylogenetic analysis of diterpene synthases and corresponding cyclized products in macro- basidiomycetes.
该课题组合成了14个Ⅰ型二萜合成酶的编码序列,并在工程化高产GGPP酿酒酵母中表达。在测定的Ⅰ型二萜合成酶中只有来自S. histurum的SteTC1具有活性。在表达SteTC1的酿酒酵母菌丝提取物的GC-MS显示有新化合物的产生。SteTC1的重组纯化蛋白能够将GGPP转化为二萜,但不能接受GPP和FPP。随后,确定新化合物结构为(-)-(R)-nephthenol (62) (Li et al. 2019)。已知化合物62是一种西松烷型二萜(cembrane),目前报道的cembrane型二萜主要来自软珊瑚和一些植物中,真菌中较少发现(Schwabe et al. 1988;Rahelivao et al. 2016)。据报道,能够产生cembrane型二萜的几种酶(来自蓖麻Ricinus communis的CAS2,来自链霉菌Streptomyces sp. SANK 60404的DtcycA和DtcycB,来自美花烟草Nicotiana sylvestris的NsCBTS2a、NsCBTS2b和NsCBTS3) (Ennajdaoui et al. 2010;Kirby et al. 2010;Meguro et al. 2014),与SteTC1相比无序列相似性,因此,SteTC1是真菌中首次发现的一种能够合成cembrane型骨架的新型二萜合成酶(Li et al. 2019)。
4 大型担子菌中UbiA型二萜合成酶
UbiA型二萜环化酶是近年发现的一种新型二萜合成酶家族(Yang et al. 2017),UbiA原型是一种参与泛醌生物合成的异戊烯基转移酶,它通过从异戊二烯基焦磷酸前体中提取焦磷酸而起作用(Li 2016)。UbiA型二萜环化酶是一种膜内酶,在生物合成泛醌的过程中催化对羟基苯甲酸的苯丙基化(Siebert et al. 1992)。这种反应是由焦磷酸的提取和苯酚的富电子邻位的亲核攻击引起的,因此类似于Ⅰ型二萜合成酶催化的反应,它们也通过焦磷酸提取催化底物反应。UbiA原型具有2个富含天冬氨酸的基序,并且也依赖于Mg2+,但整体氨基酸序列和酶结构与Ⅰ型二萜合成酶不同(Cheng & Li 2014),这表明UbiA型二萜合成酶是独立进化的。目前在担子菌中有3个UbiA型二萜合成酶的功能已被鉴定(Dickschat 2019)。
Yang et al. (2017)鉴定了H. erinaceum和C. striatus基因组中所有的GGPPS基因和邻近基因,在H. erinaceum的基因组发现了一个基因簇(命名为eri),它包括1个GGPPS和2个注释过的UbiA异戊烯基转移酶(EriF和EriG);在C. striatus基因组中也发现了一条类似的基因簇(命名为cya)。考虑到erinacines和cyathins化合物的结构,因此,他们推测eri和cya簇中UbiA相关基因可能与cyathane型二萜的生物合成有关。
为了研究EriF和EriG在猴头菌素erinacines生物合成中的作用,Yang et al. (2017)合成了这2个基因的编码区并克隆到表达载体中。在酿酒酵母中表达该基因,利用粗酶进行体外实验反应,当与GGPP和Mg2+共同孵育时,在GC-MS中观察到EriG产生新的产物,但并没有检测到EriF 的产物。为了确定化合物的结构,EriG在生产GGPP工程大肠杆菌Escherichia coli BW25113中表达,通过GC-MS分析,在提取物中检测到一种分子质量为272的高度疏水化合物。通过大规模的发酵培养,得到了3 mg/L的该化合物,核磁共振分析进一步证实该化合物为53 (Kenmoku et al. 2001) (图5)。当以GPP和FPP为底物时,未检测到产物。下一步,该课题组试图确定CyaJ和CyaP在C. striatus中的功能,但在大肠杆菌BW25113的正己烷提取物中没有检测到预期的产物。随后从C. africanus菌株中克隆了cyaP的同源基因cyaTC的完整编码序列,CyaTC的氨基酸序列与CyaP的同源性为90%,与EriG的同源性为68%,粗制的重组CyaTC酶催化GGPP生成化合物53,这些结果证明了EriG和CyaTC是负责生物合成的二萜环化酶。
5 大型担子菌中双功能型二萜合成酶
Gao课题组在推测的2个双功能型二萜合成酶中,来自Punctularia strigosozonata的PunTC和来自Serpula lacrymans的SerTC在工程酿酒酵母中异源表达并且产生了相同的环状产物,与数据图谱中的16R-ent-kaurano (63)和已在Su et al. (2017)发表的数据完全匹配。为了明确该化合物的结构,用正己烷提取了表达PunTC的酿酒酵母菌株,并进行大规模的发酵。通过硅胶柱层析等方法进一步证实该化合物为63。以上结果表明,PunTC和SerTC都是63合酶。重组纯化蛋白PunTC和SerTC分别能够将GGPP转化为二萜,而不接受GPP和FPP。表明这2个基因簇的产物是更为复杂的二萜类化合物(Li et al. 2019)。
6 结语与展望
大型担子菌除了在碳循环和生态系统功能中发挥重要作用外,还表现出生物和药理学特性(Schmidt-Dannert 2016),近年来报道了从大型担子菌中发现的重要天然产物。酶数据库中加入了功能新颖的特征酶,如参与构建cyathane二萜骨架的UbiA同源酶EriG,便于对生物合成基因簇的准确预测和注释(Chen et al. 2017)。转录组学和其他外源表达宿主可以有效地表征担子菌的生物合成基因。由于大多数担子菌属真菌在遗传转化上难以操作,因此需要开发异源表达宿主来进行蛋白表达和生物合成途径重建。除了大肠杆菌、酿酒酵母等一些较为成熟的酶功能表征系统外,一些真菌异源宿主,如米曲霉,已被证明是途径组装和重建的优秀系统。此外,现代遗传工具的应用,如CRISPR-Cas9,能够在异源表达系统中实现定点和有效的基因整合(Chen et al. 2017;Sugano et al. 2017)。
总而言之,大型担子菌中二萜类化合物具有显著的生物活性,但是其生物合成机制尚不完全清楚,仍有大量的二萜合成酶尚未被开发,对二萜合酶的基因进行分析有利于进一步阐明真菌中二萜化合物的生物合成途径,对生物合成的分子调控具有重要意义。
参考文献
Heterologous expression reveals the biosynthesis of the antibiotic pleuromutilin and generates bioactive semi-synthetic derivatives
DOI:10.1038/s41467-017-01659-1
PMID:29184068
[本文引用: 1]

The rise in antibiotic resistance is a major threat for human health. Basidiomycete fungi represent an untapped source of underexploited antimicrobials, with pleuromutilin-a diterpene produced by Clitopilus passeckerianus-being the only antibiotic from these fungi leading to commercial derivatives. Here we report genetic characterisation of the steps involved in pleuromutilin biosynthesis, through rational heterologous expression in Aspergillus oryzae coupled with isolation and detailed structural elucidation of the pathway intermediates by spectroscopic methods and comparison with synthetic standards. A. oryzae was further established as a platform for bio-conversion of chemically modified analogues of pleuromutilin intermediates, and was employed to generate a semi-synthetic pleuromutilin derivative with enhanced antibiotic activity. These studies pave the way for future characterisation of biosynthetic pathways of other basidiomycete natural products in ascomycete heterologous hosts, and open up new possibilities of further chemical modification for the growing class of potent pleuromutilin antibiotics.
Phlebiakauranol aldehyde an antifungal and cytotoxic metabolite from Punctularia atropurpurascens
DOI:10.7164/antibiotics.40.443 URL [本文引用: 1]
Concavine, an unusual diterpenic alkaloid produced by the fungus Clitocybe concava
DOI:10.1016/j.tetlet.2005.09.064 URL [本文引用: 1]
Identification and manipulation of the pleuromutilin gene cluster from Clitopilus passeckerianus for increased rapid antibiotic production
DOI:10.1038/srep25202
PMID:27143514
[本文引用: 3]

Semi-synthetic derivatives of the tricyclic diterpene antibiotic pleuromutilin from the basidiomycete Clitopilus passeckerianus are important in combatting bacterial infections in human and veterinary medicine. These compounds belong to the only new class of antibiotics for human applications, with novel mode of action and lack of cross-resistance, representing a class with great potential. Basidiomycete fungi, being dikaryotic, are not generally amenable to strain improvement. We report identification of the seven-gene pleuromutilin gene cluster and verify that using various targeted approaches aimed at increasing antibiotic production in C. passeckerianus, no improvement in yield was achieved. The seven-gene pleuromutilin cluster was reconstructed within Aspergillus oryzae giving production of pleuromutilin in an ascomycete, with a significant increase (2106%) in production. This is the first gene cluster from a basidiomycete to be successfully expressed in an ascomycete, and paves the way for the exploitation of a metabolically rich but traditionally overlooked group of fungi.
Erinacine A and related cyathane diterpenoids: molecular diversity and mechanisms underlying their neuroprotection and anticancer activities
DOI:10.1016/j.phrs.2020.104953 URL [本文引用: 1]
Secondary metabolites from higher fungi
Genomic and transcriptomic analyses reveal differential regulation of diverse terpenoid and polyketides secondary metabolites in Hericium erinaceus
DOI:10.1038/s41598-017-10376-0 URL
Structural insights into ubiquinone biosynthesis in membranes
DOI:10.1126/science.1246774
PMID:24558159
[本文引用: 1]

Biosynthesis of ubiquinones requires the intramembrane UbiA enzyme, an archetypal member of a superfamily of prenyltransferases that generates lipophilic aromatic compounds. Mutations in eukaryotic superfamily members have been linked to cardiovascular degeneration and Parkinson's disease. To understand how quinones are produced within membranes, we report the crystal structures of an archaeal UbiA in its apo and substrate-bound states at 3.3 and 3.6 angstrom resolution, respectively. The structures reveal nine transmembrane helices and an extramembrane cap domain that surround a large central cavity containing the active site. To facilitate the catalysis inside membranes, UbiA has an unusual active site that opens laterally to the lipid bilayer. Our studies illuminate general mechanisms for substrate recognition and catalysis in the UbiA superfamily and rationalize disease-related mutations in humans.
Structural and chemical biology of terpenoid cyclases
DOI:10.1021/acs.chemrev.7b00287
PMID:28841019
[本文引用: 1]

The year 2017 marks the twentieth anniversary of terpenoid cyclase structural biology: a trio of terpenoid cyclase structures reported together in 1997 were the first to set the foundation for understanding the enzymes largely responsible for the exquisite chemodiversity of more than 80000 terpenoid natural products. Terpenoid cyclases catalyze the most complex chemical reactions in biology, in that more than half of the substrate carbon atoms undergo changes in bonding and hybridization during a single enzyme-catalyzed cyclization reaction. The past two decades have witnessed structural, functional, and computational studies illuminating the modes of substrate activation that initiate the cyclization cascade, the management and manipulation of high-energy carbocation intermediates that propagate the cyclization cascade, and the chemical strategies that terminate the cyclization cascade. The role of the terpenoid cyclase as a template for catalysis is paramount to its function, and protein engineering can be used to reprogram the cyclization cascade to generate alternative and commercially important products. Here, I review key advances in terpenoid cyclase structural and chemical biology, focusing mainly on terpenoid cyclases and related prenyltransferases for which X-ray crystal structures have informed and advanced our understanding of enzyme structure and function.
Diversity and systematics of the important macrofungi in Chinese forests
Cyclization enzymes in the biosynthesis of monoterpenes, sesquiterpenes, and diterpenes
Bacterial diterpene biosynthesis
Inonotolides A-C, isopimarane diterpenoid lactones from Inonotus sinensis
DOI:10.1016/j.fitote.2018.04.006 URL [本文引用: 1]
The effects of tiamulin, a semisynthetic pleuromutilin derivative, on bacterial polypeptide chain initiation
Tiamulin, a water-soluble and highly effective semisynthetic derivative of pleuromutilin leads to the formation of physiologically inactive polypeptide chain initiation complexes which readily decompose and do not enter the phase of peptide chain elongation. Once elongation has begun it continues even in the presence of tiamulin as has been shown by measuring the formation of N-acetylphenylalanine-poly(phenylalanine). The formation of abortive initiation complexes was observed regardless of whether AcPhe-tRNA of fMet-tRNA was used as an initiator or whether artificial messengers or a natural messenger, like R17 bacteriophage RNA, was used. When this drug was acting on whole cells, it led to the disappearance of polysomes. The only structures which could be detected were of the monosome size. Therefore, polysomes seem to elongate the polypeptide chains in whole cells in the presence of this antibiotic, but since effective reinitiation is blocked, the polysome pool of the cell soon becomes depleted.
Trichome specific expression of the tobacco (Nicotiana sylvestris) cembratrien-ol synthase genes is controlled by both activating and repressing cis-regions
DOI:10.1007/s11103-010-9648-x
PMID:20495852
[本文引用: 1]

Tobacco (Nicotiana sylvestris) glandular trichomes make an attractive target for isoprenoid metabolic engineering because they produce large amounts of one type of diterpenoids, alpha- and beta-cembratrien-diols. This article describes the establishment of tools for metabolic engineering of tobacco trichomes, namely a transgenic line with strongly reduced levels of diterpenoids in the exudate and the characterization of a trichome specific promoter. The diterpene-free tobacco line was generated by silencing the major tobacco diterpene synthases, which were found to be encoded by a family of four highly similar genes (NsCBTS-2a, NsCBTS-2b, NsCBTS-3 and NsCBTS-4), one of which is a pseudogene. The promoter regions of all four CBTS genes were sequenced and found to share over 95% identity between them. Transgenic plants expressing uidA under the control of the NsCBTS-2a promoter displayed a specific pattern of GUS expression restricted exclusively to the glandular cells of the tall secretory trichomes. A series of sequential and internal deletions of the NsCBTS-2a promoter led to the identification of two cis-acting regions. The first, located between positions -589 to -479 from the transcription initiation site, conferred a broad transcriptional activation, not only in the glandular cells, but also in cells of the trichome stalk, as well as in the leaf epidermis and the root. The second region, located between positions -279 to -119, had broad repressor activity except in trichome glandular cells and is mainly responsible for the specific expression pattern of the NsCBTS-2a gene. These results establish the basis for the identification of trans-regulators required for the expression of the CBTS genes restricted to the secretory cells of the glandular trichomes.
Total syntheses of guanacastepenes N and O
The function of terpene natural products in the natural world
DOI:10.1038/nchembio.2007.5
PMID:17576428
[本文引用: 1]

As the largest class of natural products, terpenes have a variety of roles in mediating antagonistic and beneficial interactions among organisms. They defend many species of plants, animals and microorganisms against predators, pathogens and competitors, and they are involved in conveying messages to conspecifics and mutualists regarding the presence of food, mates and enemies. Despite the diversity of terpenes known, it is striking how phylogenetically distant organisms have come to use similar structures for common purposes. New natural roles undoubtedly remain to be discovered for this large class of compounds, given that such a small percentage of terpenes has been investigated so far.
Molecular networking assisted discovery and combinatorial biosynthesis of new antimicrobial pleuromutilins
DOI:10.1016/j.ejmech.2022.114713 URL [本文引用: 2]
Crinipellins A and I, two diterpenoids from the basidiomycete fungus Crinipellis rhizomaticola, as potential natural fungicides
DOI:10.3390/molecules23092377 URL [本文引用: 1]
The mode of action of pleuromutilin derivatives
DOI:10.1111/j.1432-1033.1975.tb03976.x URL [本文引用: 1]
The mode of action of pleuromutilin derivatives
Inhibition of nitric oxide production in RAW 264.7 macrophages by diterpenoids from Phellinus pini
DOI:10.1007/s12272-011-0608-z URL [本文引用: 1]
The physiology of production of the antibiotic cyathin by Cyathus helenae
Antibiotic substances from basidiomycetes:Ⅷ. Pleurotus multilus (Fr.) Sacc. and Pleurotus passeckerianus Pilat
Erinacines A, B and C, strong stimulators of nerve growth factor (NGF)-synthesis, from the mycelia of Hericium erinaceum
DOI:10.1016/S0040-4039(00)76760-8 URL [本文引用: 1]
Isolation of (-)-cyatha- 3,12-diene, a common biosynthetic intermediate of cyathane diterpenoids, from an erinacine-producing basidiomycete, Hericium erinaceum, and its formation in a cell-free system
DOI:10.1016/S0040-4039(01)01550-7 URL [本文引用: 1]
Erinacol (cyatha-3,12-dien-14β-ol) and 11-O-acetylcyathin A(3), new cyathane metabolites from an erinacine Q-producing Hericium erinaceum
DOI:10.1271/bbb.68.1786 URL [本文引用: 1]
Progress in the chemistry of organic natural products 106
Cloning of casbene and neocembrene synthases from Euphorbiaceae plants and expression in Saccharomyces cerevisiae
DOI:10.1016/j.phytochem.2010.06.001 URL [本文引用: 1]
Botryane, noreudesmane and abietane terpenoids from the ascomycete Hypoxylon rickii
DOI:S0031-9422(15)30015-7
PMID:26071840
[本文引用: 1]

In the course of our screening for new bioactive natural products, a culture of Hypoxylon rickii, a xylariaceous ascomycete collected from the Caribbean island Martinique, was identified as extraordinary prolific producer of secondary metabolites. Ten metabolites of terpenoid origin were isolated from submerged cultures of this species by preparative HPLC. Their structures were elucidated using spectral techniques including 2D NMR and HRESIMS. Three of the compounds were elucidated as new botryanes (1-3) along with three known ones, i.e. (3aS)-3a,5,5,8-tetramethyl-3,3a,4,5-tetrahydro-1H-cyclopenta[de]isochromen-1-one (4), (3aS,8R)-3a,5,5,8-tetramethyl-3,3a,4,5,7,8-hexahydro-1H-cyclopenta[de]isochromen-1-one (5) and botryenanol (6). Further three new sesquiterpenoids featured a 14-noreudesmane-type skeleton and were named hypoxylan A-C (7-9); the diterpenoid rickitin A (10) contains an abietane-type backbone. Compounds 1, 2, 3, 7, and 10 showed cytotoxic effects against murine cells. Copyright © 2015 Elsevier Ltd. All rights reserved.
Mevalonate and nonmevalonate pathways for the biosynthesis of isoprene units
DOI:10.1271/bbb.66.1619
PMID:12353619
[本文引用: 1]

Isoprenoids are synthesized by consecutive condensations of their five-carbon precursor, isopentenyl diphosphate, to its isomer, dimethylallyl diphosphate. Two pathways for these precursors are known. One is the mevalonate pathway, which operates in eucaryotes, archaebacteria, and cytosols of higher plants. The other is a recently discovered pathway, the nonmevalonate pathway, which is used by many eubacteria, green algae, and chloroplasts of higher plants. To date, five reaction steps in this new pathway and their corresponding enzymes have been identified. EC numbers of these enzymes have been assigned by the Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB) and are available at http://www.chem.qmw.ac.uk/iubmb/enzyme/reaction/terp/nonMVA.html.
Inhibition of human neutrophil elastase by labdane diterpenes from the fruiting bodies of Ramaria formosa
DOI:10.1080/09168451.2015.1065168 URL [本文引用: 1]
Research progress on biosynthesis pathway of pentacyclic triterpenoids in plants
Bringing bioactive compounds into membranes: the UbiA superfamily of intramembrane aromatic prenyltransferases
DOI:S0968-0004(16)00023-2
PMID:26922674
[本文引用: 1]

The UbiA superfamily of intramembrane prenyltransferases catalyzes a key biosynthetic step in the production of ubiquinones, menaquinones, plastoquinones, hemes, chlorophylls, vitamin E, and structural lipids. These lipophilic compounds serve as electron and proton carriers for cellular respiration and photosynthesis, as antioxidants to reduce cell damage, and as structural components of microbial cell walls and membranes. This article reviews the biological functions and enzymatic activities of representative members of the superfamily, focusing on the remarkable recent research progress revealing that the UbiA superfamily is centrally implicated in several important physiological processes and human diseases. Because prenyltransferases in this superfamily have distinctive substrate preferences, two recent crystal structures are compared to illuminate the general mechanism for substrate recognition.Copyright © 2016 Elsevier Ltd. All rights reserved.
Rapid discovery and functional characterization of diterpene synthases from basidiomycete fungi by genome mining
DOI:10.1016/j.fgb.2019.03.007 URL [本文引用: 6]
Chemical constituents from Inonotus obliquus and their biological activities
DOI:10.1021/np400552w URL [本文引用: 1]
Efficient reconstitution of basidiomycota diterpene erinacine gene cluster in Ascomycota host Aspergillus oryzae based on genomic DNA sequences
DOI:10.1021/jacs.9b08935 URL [本文引用: 2]
Advance in fungal diterpene cyclase: a review
PMID:21268887

Diterpenoid is a huge group of nature products isolated from plants and fungi. Diterpene cyclase, which is responsible for the diterpene carbon skeleton formation from geranylgeranyl diphosphate (GGPP), is a key enzyme in the biosynthetic pathway of diterpene. The specificity of diterpene cyclase in different species results in structural diversity and bioactivity variety of diterpenoid. Isolation and characterization of the diterpene cyclase in various species will facilitate studies on the biosynthesis and regulation of diterpenoid in future. Compared to plant diterpenoids, few fungal diterpenoid and diterpene cyclase were studied. This article reviews the research advancement of fungal diterpene cyclase in recent years, especially describes the biosynthesis pathway of diterpenoid, the characteristics and cloning strategies of fungal diterpene cyclase, and the metabolic engineering of diterpenoid.
Guanacastane-type diterpenoids with cytotoxic activity from Coprinus plicatilis
DOI:10.1016/j.bmcl.2012.06.006 URL [本文引用: 1]
Psathyrins: antibacterial diterpenoids from Psathyrella candolleana
DOI:10.1021/acs.jnatprod.0c00269 URL [本文引用: 1]
Reconstitution of biosynthetic pathway for mushroom-derived cyathane diterpenes in yeast and generation of new “non-natural” analogues - science direct
DOI:10.1016/j.apsb.2021.04.014 URL [本文引用: 1]
Corrigendum: identification and characterization of bacterial diterpene cyclases that synthesize the cembrane skeleton
DOI:10.1002/cbic.201200651 URL [本文引用: 1]
A pathway where polyprenyl diphosphate elongates in prenyltransferase. Insight into a common mechanism of chain length determination of prenyltransferases
DOI:10.1074/jbc.273.41.26705 URL [本文引用: 1]
Guanacastane-type diterpenoids from Coprinus radians
DOI:10.1016/j.phytochem.2012.03.002 URL [本文引用: 1]
Antimicrobial activity of the pleuromutilin antibiotic BC-3781 against bacterial pathogens isolated in the SENTRY Antimicrobial Surveillance Program in 2010
DOI:10.1128/AAC.00358-13
PMID:23836172
[本文引用: 1]

BC-3781 is a novel semisynthetic pleuromutilin antibiotic inhibiting bacterial protein synthesis. BC-3781 has completed a phase 2 clinical trial in acute bacterial skin and skin structure infections (ABSSSI). Its antibacterial spectrum additionally covers the predominant pathogens causing community-acquired bacterial pneumonia (CABP). In this study, the antibacterial activity of BC-3781 was evaluated against a contemporary collection of 10,035 bacterial isolates predominately causing ABSSSI and CABP, among other infections, collected within the SENTRY Antimicrobial Surveillance Program worldwide in 2010. BC-3781 exhibited potent activity against organisms commonly isolated from ABSSSI such as Staphylococcus aureus (MIC50/90, 0.12/0.12 μg/ml; 99.8% inhibited at ≤0.5 μg/ml), beta-hemolytic streptococci (MIC50/90, 0.03/0.03 μg/ml; 99.3% inhibited at ≤0.5 μg/ml), and coagulase-negative staphylococci (CoNS; MIC50/90, 0.06/0.12 μg/ml; 97.8% inhibited at ≤1 μg/ml). BC-3781 displayed similar MIC distributions among methicillin-susceptible (MSSA) and methicillin-resistant (MRSA) S. aureus strains. BC-3781 was also active against Enterococcus faecium, with 76.3% of vancomycin-susceptible and 97.0% of vancomycin-resistant isolates being inhibited at BC-3781 concentrations of ≤1 μg/ml. Beta-hemolytic and viridans group streptococci were highly susceptible to BC-3781, with 99.3% and 96.7% of isolates inhibited at ≤0.5 μg/ml, respectively. Further, activity of BC-3781 against Streptococcus pneumoniae (MIC50/90, 0.12/0.25 μg/ml), Haemophilus influenzae (MIC50/90, 1/2 μg/ml), and Moraxella catarrhalis (MIC50/90, 0.12/0.25 μg/ml) was not negatively influenced by β-lactamase production or resistance to other antimicrobial classes tested. In all, BC-3781 displayed a very potent antibacterial profile including the most prevalent bacterial pathogens causing ABSSSI and CABP, thus warranting further clinical development of this antibiotic in these and possibly other indications.
Research progress on cyathanes from macrofungi
The pleuromutilin drugs tiamulin and valnemulin bind to the RNA at the peptidyl transferase centre on the ribosome
The pleuromutilin antibiotic derivatives, tiamulin and valnemulin, inhibit protein synthesis by binding to the 50S ribosomal subunit of bacteria. The action and binding site of tiamulin and valnemulin was further characterized on Escherichia coli ribosomes. It was revealed that these drugs are strong inhibitors of peptidyl transferase and interact with domain V of 23S RNA, giving clear chemical footprints at nucleotides A2058-9, U2506 and U2584-5. Most of these nucleotides are highly conserved phylogenetically and functionally important, and all of them are at or near the peptidyl transferase centre and have been associated with binding of several antibiotics. Competitive footprinting shows that tiamulin and valnemulin can bind concurrently with the macrolide erythromycin but compete with the macrolide carbomycin, which is a peptidyl transferase inhibitor. We infer from these and previous results that tiamulin and valnemulin interact with the rRNA in the peptidyl transferase slot on the ribosomes in which they prevent the correct positioning of the CCA-ends of tRNAs for peptide transfer.
Traversing the fungal terpenome
DOI:10.1039/c4np00075g
PMID:25171145
[本文引用: 1]

Fungi (Ascomycota and Basidiomycota) are prolific producers of structurally diverse terpenoid compounds. Classes of terpenoids identified in fungi include the sesqui-, di- and triterpenoids. Biosynthetic pathways and enzymes to terpenoids from each of these classes have been described. These typically involve the scaffold generating terpene synthases and cyclases, and scaffold tailoring enzymes such as e.g. cytochrome P450 monoxygenases, NAD(P)+ and flavin dependent oxidoreductases, and various group transferases that generate the final bioactive structures. The biosynthesis of several sesquiterpenoid mycotoxins and bioactive diterpenoids has been well-studied in Ascomycota (e.g. filamentous fungi). Little is known about the terpenoid biosynthetic pathways in Basidiomycota (e.g. mushroom forming fungi), although they produce a huge diversity of terpenoid natural products. Specifically, many trans-humulyl cation derived sesquiterpenoid natural products with potent bioactivities have been isolated. Biosynthetic gene clusters responsible for the production of trans-humulyl cation derived protoilludanes, and other sesquiterpenoids, can be rapidly identified by genome sequencing and bioinformatic methods. Genome mining combined with heterologous biosynthetic pathway refactoring has the potential to facilitate discovery and production of pharmaceutically relevant fungal terpenoids.
Chemical constituents of the soft corals Sinularia vanderlandi and Sinularia gravis from the coast of Madagascar
Pimarane diterpenes: natural source, stereochemical configuration, and biological activity
DOI:10.1002/chir.23009
PMID:30153350
[本文引用: 1]

Plants and fungi are seemingly inexhaustible sources of interesting natural products with remarkable structural and biological diversity. One of the most important groups is the terpenes, ubiquitous natural products that are generated by 2 now well-established biosynthetic pathways: the older mevalonate and the more recently discovered 1-deoxyxylulose-5-phosphate. Among the diterpenes, the pimarane diterpenes are a very representative subgroup with several and interesting biological activities resulting from different functional group modifications. In this review, we outline the method of their structure determination, mainly spectroscopic results, their absolute configuration, and structure-activity relationships, were reported, as well as the mode of action for selected examples from plants, marine organisms, and fungi. The pimarane, isopimarane, and ent-pimarane diterpenes covered in this review have a wide range of biological activities including antimicrobial, antifungal, antiviral, phytotoxic, phytoalexin, cytotoxicity, and antispasmodic and relaxant effects.© 2018 Wiley Periodicals, Inc.
Anti-inflammatory tetraquinane diterpenoids from a Crinipellis species
DOI:10.1016/j.bmc.2016.11.016 URL [本文引用: 1]
Biocatalytic portfolio of basidiomycota
DOI:10.1016/j.cbpa.2016.01.002
PMID:26812494
[本文引用: 1]

Basidiomycota fungi have received little attention for applications in biocatalysis and biotechnology and remain greatly understudied despite their importance for carbon recycling, ecosystem functioning and medicinal properties. The steady influx of genome data has facilitated detailed studies aimed at understanding the evolution and function of fungal lignocellulose degradation. These studies and recent explorations into the secondary metabolomes have uncovered large portfolios of enzymes useful for biocatalysis and biosynthesis. This review will provide an overview of the biocatalytic repertoires of Basidiomycota characterized to date with the hope of motivation more research into the chemical toolkits of this diverse group of fungi.Copyright © 2016 Elsevier Ltd. All rights reserved.
Ubiquinone biosynthesis cloning of the genes coding for chorismate pyruvate-lyase and 4-hydroxybenzoate octaprenyl transferase from Escherichia coli
Chorismate pyruvate-lyase activity was detected in extracts of Escherichia coli. 4-Hydroxybenzoate was identified as the product of the enzymatic reaction by chemical derivatization and GC-MS analysis. The ubiC gene, coding for the chorismate pyruvate-lyase, was cloned and sequenced. The molecular weight of the gene product was calculated as 18,776 Da and confirmed by expression of the protein in E. coli minicells. The ubiA gene, coding for the 4-hydroxybenzoate octaprenyl transferase, was identified by sequence homology and complementation of a ubiA- strain. It is located directly downstream of ubiC in a typical operon structure.
Probing the single key amino acid responsible for the novel catalytic function of ent-kaurene oxidase supported by NADPH-cytochrome P 450 Reductases in Tripterygium wilfordii
DOI:10.3389/fpls.2017.01756 URL [本文引用: 1]
Genome editing in the mushroom- forming basidiomycete Coprinopsis cinerea, optimized by a high-throughput transformation system
DOI:10.1038/s41598-017-00883-5 URL [本文引用: 1]
A comparison of triterpenoids and polysaccharides in 13 species of wild Ganoderma
Erinacine A-enriched Hericium erinaceus mycelium ameliorates Alzheimer’s disease-related pathologies in APPswe/PS1dE 9 transgenic mice
DOI:10.1186/s12929-015-0217-0 URL [本文引用: 1]
A review of research on the active secondary metabolites of Hericium species
An efficient strategy to enhance triterpenoid production by liquid superficial-static culture (LSSC) of Ganoderma lingzhi
Eryngiolide A, a cytotoxic macrocyclic diterpenoid with an unusual cyclododecane core skeleton produced by the edible mushroom Pleurotus eryngii
DOI:10.1021/ol301519m URL [本文引用: 1]
An abietane diterpene and a sterol from fungus Phellinus igniarius
A checklist of edible ectomycorrhizal mushrooms in China
Assessment of the threatened status of macro-basidiomycetes in China
Global diversity and systematics of Hymenochaetaceae with poroid hymenophore
DOI:10.1007/s13225-021-00496-4 URL [本文引用: 1]
Resource diversity of Chinese macrofungi: edible, medicinal and poisonous species
DOI:10.1007/s13225-019-00432-7 URL [本文引用: 1]
Isolation and identification of a new anti- inflammatory cyathane diterpenoid from the medicinal fungus Cyathus hookeri Berk
DOI:10.1016/j.fitote.2013.03.002 URL [本文引用: 1]
Biosynthetic machinery of diterpene pleuromutilin isolated from basidiomycete fungi
DOI:10.1002/cbic.201700434
PMID:28924980
[本文引用: 2]

The diterpene pleuromutilin is a ribosome-targeting antibiotic isolated from basidiomycete fungi, such as Clitopilus pseudo-pinsitus. The functional characterization of all biosynthetic enzymes involved in pleuromutilin biosynthesis is reported and a biosynthetic pathway proposed. In vitro enzymatic reactions and mutational analysis revealed that a labdane-related diterpene synthase, Ple3, catalyzed two rounds of cyclization from geranylgeranyl diphosphate to premutilin possessing a characteristic 5-6-8-tricyclic carbon skeleton. Biotransformation experiments utilizing Aspergillus oryzae transformants possessing modification enzyme genes allowed the biosynthetic pathway from premutilin to pleuromutilin to be proposed. The present study sets the stage for the enzymatic synthesis of natural products isolated from basidiomycete fungi, which are a prolific source of structurally diverse and biologically active terpenoids.© 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Biochemical characterization of the interactions of the novel pleuromutilin derivative retapamulin with bacterial ribosomes
Retapamulin is a semisynthetic pleuromutilin derivative being developed as a topical antibiotic for treating bacterial infections of the skin. It is potent in vitro against susceptible and multidrug-resistant organisms commonly associated with bacterial skin infections. We report detailed mode of action studies demonstrating that retapamulin binds to the bacterial ribosome with high affinity, inhibits ribosomal peptidyl transferase activity, and partially inhibits the binding of the initiator tRNA substrate to the ribosomal P-site. Taken together, these data distinguish the mode of action of retapamulin from that of other classes of antibiotics. This unique mode of action may explain the lack of clinically relevant, target-specific cross-resistance of retapamulin with antibacterials in current use.
Discovery and characterization of a new family of diterpene cyclases in bacteria and fungi
The biosynthesis of diterpenoids from plants
Five new guanacastane-type diterpenes from cultures of the fungus Psathyrella candolleana
DOI:10.1007/s13659-014-0020-8 URL [本文引用: 1]
New cyathane diterpenoids with neurotrophic and anti-neuroinflammatory activity from the bird’s nest fungus Cyathus africanus
DOI:10.1016/j.fitote.2019.02.013 URL [本文引用: 1]
Signal transduction and gene expression analysis of triterpenoid acid synthesis induced by 9,10- cyclomethylheptadecanoic acid in Ganoderma lingzhi
Terpenoids with alpha-glucosidase inhibitory activity from the submerged culture of Inonotus obliquus
DOI:10.1016/j.phytochem.2014.09.022 URL [本文引用: 1]
Studies on the neurotrophic action of cyathane diterpenoids and meroteroenoids in high fungi
Diterpenes specially produced by fungi: structures, biological activities, and biosynthesis (2010-2020)
A viscidane diterpene and polyacetylenes from cultures of Hypsizygus marmoreus
DOI:10.1007/s13659-015-0058-2 URL [本文引用: 1]
The abietane type diterpenes from Isodon (Bl.) Hassk
Two new cleistanthane diterpenes and a new isocoumarine from cultures of the basidiomycete Albatrellus confluens
中国森林大型真菌重要类群多样性和系统学研究
9,10-环甲基十七烷酸诱导灵芝三萜酸合成的信号转导和基因表达分析