 PDF(615 KB)
PDF(615 KB)


油酸促进灵芝三萜液态深层发酵的工艺研究及规模化验证
苏晓薇,唐庆九,张劲松,冯娜,王金艳,周帅,冯杰,俞苓
菌物学报 ›› 2021, Vol. 40 ›› Issue (9) : 2445-2460.
 PDF(615 KB)
PDF(615 KB)
 PDF(615 KB)
PDF(615 KB)
油酸促进灵芝三萜液态深层发酵的工艺研究及规模化验证
 ({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}
({{custom_author.role_cn}}), {{javascript:window.custom_author_cn_index++;}}Synthesis of triterpenes in liquid submerged fermentation of Ganoderma lingzhi promoted by oleic acid
 ({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}
({{custom_author.role_en}}), {{javascript:window.custom_author_en_index++;}}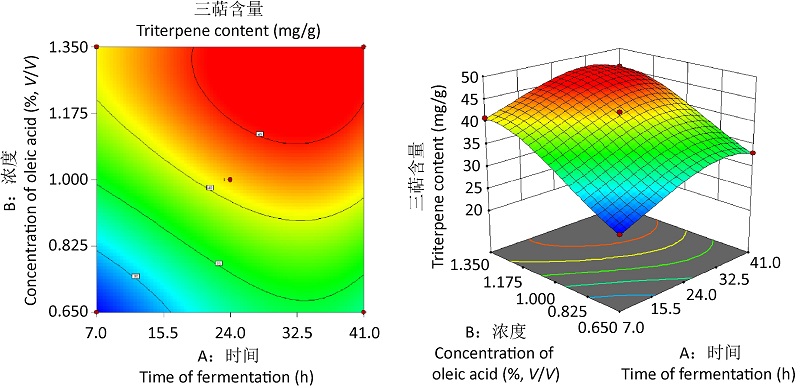
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |