糙皮侧耳Pleurotus ostreatus (Jacq.) P. Kumm.,是世界上栽培面积最广泛的食用菌之一(Nam et al. 2018 )。它以丰富的营养如碳水化合物、蛋白质、矿物质和维生素,显著的抗氧化和保健功能而受到消费者的青睐(Jayakumaret al. 2008 ;Wuet al. 2019 ),是重要的食药用真菌(戴玉成和杨祝良2008;戴玉成等2010)。糙皮侧耳适应性强,可以在很多种农业副产物上生长,因此其年产量持续快速增长(Sánchez 2010)。目前,糙皮侧耳的主要栽培技术有3种:生料栽培、熟料栽培和发酵料栽培(Hernándezet al. 2003 )。与生料和熟料栽培相比,发酵料栽培糙皮侧耳由于具有低污染、低成本、工艺简单和经济效益高的优点在世界范围内得到广泛应用(Hernándezet al. 2003 )。发酵料是以玉米芯、秸秆等农作物副产品为主要原料,麸皮、尿素等为辅助原料,经短期微生物好氧发酵后制成(Konget al. 2020 )。微生物在发酵料制备过程中起着重要作用,微生物群落动态变化影响有机物的降解,而有机物的降解决定了发酵的成熟度(Wei et al. 2007 )。发酵结束后,这些微生物依然存在于发酵料中,有可能与食用菌相伴而生并对其生长发育产生一定的作用。甚至有些食用菌必须与其他微生物共生才能完成正常的生长发育,产生子实体,继而完成繁殖,否则食用菌产量会受到影响以至减产,甚至绝收(Carrasco et al. 2020 )。微生物将培养料中的复杂有机质(纤维素、半纤维素、木质素等)进行降解,使其变成相对简单、易于利用的物质从而被食用菌吸收利用。某些特定微生物(菌根辅助细菌)可以刺激菌根真菌的生长,同时促进共生合作的建立;通过次级代谢产物(吲哚乙酸)促进菌丝的生长;通过消耗可挥发性物质(乙烯和1-辛烯-3-醇)来诱导蘑菇原基分化(蘑菇从营养生长转变为生殖生长);通过分泌抗生素来抑制竞争性真菌(Carrasco & Preston 2020)。
宏基因组学(metagenomics),又称元基因组学,是以特定环境中的整个微生物群落作为研究对象,无需分离培养,直接从样品中提取全部微生物的DNA,构建宏基因组文库。利用基因组学的研究策略分析环境微生物的群落结构、物种分类、系统进化、基因功能及代谢网络等,目前已广泛应用于微生物领域(Konget al. 2020 )。代谢组学(metabonomics)是继基因组学、转录组学和蛋白质组学后出现的新兴组学技术,是系统生物学的组成部分(雷露等 2020),旨在研究生物整体、器官或组织的内源性代谢物的代谢途径、受内外在因素的影响以及随时间变化的规律(Bolten et al. 2007 ;Fiehn 2009;Somerville & Proctor 2009)。其中,非靶向代谢组学(untargeted metabolomics)是以发现为目的,运用现代检测技术无偏向性地对生物样本所包含的尽可能多的代谢产物进行检测分析,最大程度呈现总的代谢物信息(Xie et al. 2020 )。目前,代谢组学技术已经开始应用于农产品生长、贮藏过程中代谢物合成转化规律和变化趋势的相关研究(刘平香 2020)。
在我们前期的研究中发现:糙皮侧耳发酵料制备过程中,变形菌门Proteobacteria在早期阶段(T1)占优势(相对丰度35.67%- 44.10%),在高温阶段(T2,T3),厚壁菌门Firmicutes成为新的优势门(相对丰度41.50%- 48.52%),而在堆肥后期(T4、T5),变形菌门重新成为优势门(相对丰度33.55%-36.89%)。这些微生物对发酵过程中植物秸秆中木质纤维素的降解起着至关重要的作用。Pearson相关分析表明,发酵料制备过程中,发酵料的理化性质变化与微生物菌落的组成变化具有显著的关系(Konget al. 2020 )。发酵结束后,这些微生物依然存在于发酵料中并有可能与糙皮侧耳相伴而生。那么,微生物的种类将随着糙皮侧耳的生长发生怎样变化?其代谢产物主要有哪些?这些微生物和代谢产物在糙皮侧耳的生长发育过程中起什么作用?目前还未有相关报道。因此,本研究将通过宏基因组测序的方法对糙皮侧耳生长不同时期发酵料中的微生物种类进行分析;采用代谢组学的方法对发酵料中的代谢产物进行鉴定;进而通过SPSS软件对糙皮侧耳、微生物、代谢产物进行Pearson相关性分析,深入了解微生物与食用菌的相互作用,为改进现有栽培方法、提高食用菌的生物学效率提供理论支持。
1 材料与方法
1.1 供试材料
1.1.1 菌株:糙皮侧耳Pleurotus ostreatus由河南省食用菌种质资源库提供。
1.1.2 发酵料:玉米芯84%,麸皮10%,石灰5%,尿素1%,含水量68%。
1.1.3 试剂:玉米芯、麸皮、石灰、尿素均购自本地农贸市场;LC-MC级甲醇、水、甲酸、醋酸铵(CNW Technologies GmbH,Germany);FastDNA SPIN Kit for Soil(MP,USA);AxyPrep DNA Purification Kit(AXYGEN,Inc.)。
1.1.4 仪器:Nano Drop ND-1000 UV-vis分光光度计(Thermo Scientific,Rockwood,TN,USA);BSA124S-CW天平(Sartorius);1290 UHPLC色谱仪(Agilent Technologies Inc.,USA);Triple TOF 6600质谱仪(AB Sciex Pte. Ltd.,USA);D3024R低温离心机(Scilogex,USA);JXFSTPRP-24全自动样品快速研磨仪(上海净信实业发展有限公司);普析GWB-1纯水仪(北京普析通用仪器有限责任公司);KS-7200DV超声仪(昆山洁力美超声仪器有限公司);伯乐T100TMPCR仪(Bio-Rad Laboratories,Inc.)。
1.2 糙皮侧耳栽培和取样
根据Kong et al.(2020)的报道,制备糙皮侧耳发酵料。按照河南省地方标准《糙皮侧耳发酵料栽培技术规程》(DB41/T1211-2016)进行糙皮侧耳的栽培。分别在培养料发酵完成期(T1)、糙皮侧耳菌丝定植期(T2)、发菌中期(T3)、发菌末期(T4)、子实体七分熟期(T5)5个时期进行取样。每个生长时期各随机选取9个不同的栽培袋,将其中的样品发酵料揉碎混合均匀后分成两份置于-80℃冰箱中保存,分别用于后续宏基因测序及代谢物分析。
1.3 宏基因组测序
1.3.1 DNA提取和PCR扩增:发酵料基因组DNA的提取采用试剂盒FastDNA SPIN Kit for Soil(MP,USA)。提取的DNA用1.0%琼脂糖凝胶电泳检测,DNA的浓度和质量采用Nano Drop ND-1000 UV-vis分光光度计(Thermo Scientific,Rockwood,TN,USA)测定。微生物DNA的扩增区域为16S rRNA的V3-V4区,通用引物为338F(5’-ACTCCTACGGGAGGCAGC AG-3’)以及(5’-GGACTACHVGGGTWTCTAAT-3’)806R,PCR扩增采用20μL的反应体系,反应参数为:1×(95℃ 3min);30×(95℃ 30s,55℃ 30s,72℃ 45s);1×(72℃ 10min);4℃直到用户停止。PCR产物收集和纯化采用试剂盒AxyPrep DNA purification kit(AXYGEN,Inc.)。高通量测序经武汉康测生物科技有限公司,在MiSeq PE300 platform(Illumina,USA)上完成。
1.3.2 宏基因组分析:使用软件Trimmomatic(version 0.33)对raw reads进行质量修剪和过滤,以获得high-quality reads。利用拼接软件Megahit(version 1.1.2),采用不同大小的K-mer(范围57-137,步长为10)对过滤后的数据进行组装,选择其中最优组装结果。采用Prokka(version 1.13.3 2)软件预测拼接contigs的开放阅读框(ORF),并从中筛选出长度大于100bp的基因,将其翻译成对应的氨基酸序列(王晴 2019)。采用CD-HIT(version 4.7)(
1.4 代谢组学分析
1.4.1 代谢物的提取:称取20mg液氮研磨的发酵料样本,置于1.5mL离心管中,加入500μL的80%甲醇水溶液;涡旋振荡30s,冰浴静置5min,15 000×g离心20min;取一定量上清加LC-MS级水稀释甲醇含量为53%;15 000×g 4℃离心20min,收集上清液上机进行LC-MS分析。
1.4.2 色谱条件:采用Hypesil Gold column色谱柱(100mm×2.1mm,1.9μm);正模式:流动相A:0.1%甲酸,流动相B:甲醇;负模式:流动相A:5mmol/L醋酸铵,pH 9.0,流动相B:甲醇。色谱梯度洗脱程序:0-1.5min,2% B;1.5-12min,2%-100% B;12-14min,100% B;14-14.1min,100%-2% B;14.1%-17%,2% B。柱温40℃;流速为0.2mL/min。
1.4.3 质谱条件:扫描范围选择m/z 100- 1 500;ESI源的设置如下:喷雾电压(spray voltage):3.2kV;鞘气(sheath gas)流速:40arb;辅气流速(aux gas):10arb;毛细管温度(capillary temperature):320℃;极性:正离子(positive)、负离子(negative);MS/MS二级扫描为data-dependent scans。
1.4.4 成分鉴定和分析:将下机数据(.raw)文件导入CD搜库软件中,进行保留时间、质荷比等参数的简单筛选,然后对不同样品根据保留时间偏差0.2min和质量偏差5进行峰对齐,使鉴定更准确,随后根据设置的质量偏差5、信号强度偏差30%、信噪比3、最小信号强度100 000、加和离子等信息进行峰提取,同时对峰面积进行定量,再整合目标离子,然后通过分子离子峰和碎片离子进行分子式的预测并与mzCloud(https://www. mzcloud.org/)、mzVault和Masslist数据库进行比对,用blank样本去除背景离子,并对定量结果进行归一化,最后得到数据的鉴定和定量结果。采用SIMCA软件(version 14.1,sartorius stedim data analytics AB,Umea,Sweden)对数据进行主成分(principal component analysis,PCA)和正交偏最小二乘判别分析(orthogonal partial least squares discriminant analysis,OPLS-DA)(张萌等 2020)。利用R语言(version 3.1.2)的vegan包绘制热图,筛选差异代谢物。采用MetaboAnalyst 4.0(https://www.metaboana lyst.ca)对差异代谢物富集通路进行分析。
1.5 数据处理分析
试验数据均采用Excel 2016进行记录整理,采用SPSS(version 20.0)进行Pearson相关性分析以及单因素ANOVA检验比较平均值,统计分析结果(P<0.05)为差异性显著,试验结果用平均值和SEM表示。采用SPSS(version 20.0)进行。
2 结果与分析
2.1 糙皮侧耳生长期间发酵料中微生物种群的演替
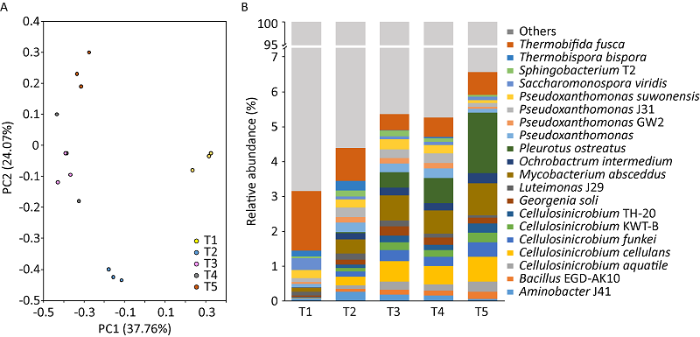
为了确定糙皮侧耳生长过程中发酵料中微生物群落的遗传信息,对糙皮侧耳5个典型生长时期的发酵料样品采用宏基因组测序方法进行了分析研究。PCA分析中,样品组成越相似,在PCA图中的距离越近,不同环境或不同处理下的样品可能表现出分散和聚集的分布情况,从而判断相同条件下的样品组成是否具有相似性。发酵料样品中的微生物分成4组,其中T3和T4聚为一组,而T1、T2和T5各为一组(图1A),表明糙皮侧耳不同生长时期发酵料中微生物群落组成具有显著差异。
图1
图1
糙皮侧耳不同生长时期发酵料中微生物群落的PCA得分图(A)和在种水平上的分类结构(B)
T1、T2、T3、T4和T5分别表示发酵完成(第0天,接种前)、菌丝定植(第3天)、发菌中期(第10天)、发菌末期(第20天,原体形成前)和P.ostreatus第一茬菇(第30天,子实体发育)
Fig. 1
PCA scores (A) and taxonomic structure at species level (B) of the bacterial communities in culture compost at the different growth stages of Pleurotus ostreatus.
T1: Composting was completed (day 0, before spawning); T2: Mycelial colonization (day 3); T3: Mid-incubation (day 10); T4: End-incubation (day 20, before primordial formation); T5: First P.ostreatusfructification (day 30, fruiting body development).
相对丰度较大的优势物种可以反映样本本身的特性或是样品与周围环境之间的关系(Zhou et al. 2019 )。群落组成分析结果表明,糙皮侧耳不同生长阶段,不同物种(top 20)的相对丰度具有明显的变化(图1B)。与预期相符,随着种植时间的增加,糙皮侧耳的相对丰度不断增加(从0.00040%增加到1.74%)。并且,随着糙皮侧耳的生长,BacillusEGD-AK10、Cellulosinicrobium aquatile、Cellulosinicrobium cellulans、Cellulosinicrobium funkei、CellulosinicrobiumKWT-B、CellulosinicrobiumTH-20、Mycobacterium abscessus、Ochrobactrum intermedium的相对丰度不断增加,表明这些物种与糙皮侧耳之间可能存在一定的协同作用。据报道,这些属的微生物具有较好的木质纤维素降解能力,能够将复杂碳水化合物转化为易于糙皮侧耳菌丝吸收和利用的单糖(Carrasco & Preston 2020)。据报道,作为厚壁菌门的一员,芽孢杆菌属Bacillus的多个种对有机物的降解和有害真菌的抑制具有显著的作用(Kong et al. 2020 )。从常规栽培料中分离的Bacillus spp.可以通过诱导漆酶的产生来抑制哈茨木霉Trichoderma harzianum,优化糙皮侧耳的生长(Zhang et al. 2020 )。纤维菌属Cellulosimicrobium的细菌可以产生多酶复合物,如木聚糖酶组(xylanosomes),促进木质素和半纤维素的分解(Abhisheket al. 2016 ),为糙皮侧耳的生长提供易于利用的小分子物质。Ochrobactrum intermedium对敌敌畏、除草剂双草醚和草甘膦具有较好的降解作用,具有提高发酵料和糙皮侧耳质量的潜在作用(Ahmad et al. 2018 )。假黄色单胞菌属Pseudoxanthomonas的细菌在糙皮侧耳的营养生长阶段表现出较高的相对丰度,该属对复杂碳源具有较强的降解作用(Carrasco & Preston 2020)。Thermobifida fusca在糙皮侧耳整个生长过程中都呈现较高的相对丰度,其是一种好氧、嗜热的细菌,能产生耐热的胞外纤维素酶和漆酶,对木质纤维素具有较好的降解作用(Chen et al. 2013 )。随着糙皮侧耳的生长,Luteimonas J29、Pseudoxanthomonas suwonensis、Thermobispora bispora等细菌在发酵料中的相对丰度显著降低,这可能是由于糙皮侧耳菌丝对某些特定的微生物具有显著的负影响。该结果与Voset al.(2017)的研究一致,通过对比接种和未接种双孢蘑菇的培养料发现,双孢蘑菇菌丝的存在会显著降低细菌群落的整体丰度。蘑菇代谢物如糖类、氨基酸、硫醇和各种酶等以及其生长条件均会对培养料中的细菌群落产生显著影响(Vos et al. 2017 ;Carrasco & Preston 2020)。这些微生物丰度的变化趋势意味着糙皮侧耳生长过程中发酵料中可能存在一个适合糙皮侧耳生长的特殊微生境,其中特定的细菌被选择。因此,微生物群落结构的演变可以解释为发酵料中特定的微生物对木质纤维素生物质和糙皮侧耳的独特适应。
2.2 糙皮侧耳不同生长时期发酵料中微生物种群组成的差异
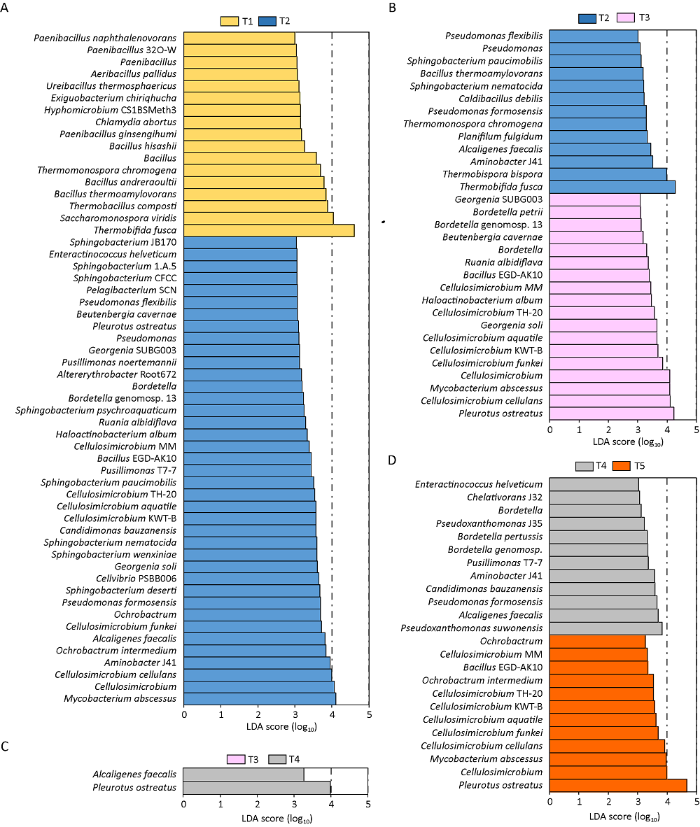
采用LEfSe(linear discriminant analysis effect size)分析和确定了糙皮侧耳不同生长阶段发酵料中相对丰度具有显著差异的微生物(图2)。LDA阈值为3.0时,在T1和T2、T2和T3、T3和T4、T4和T5之间分别有56种、31种、2种和24种微生物的相对丰度存在显著差异。结果表明,T1和T2时期发酵料中的微生物组成差异最大,其次是T2和T3,T3和T4差异最小。糙皮侧耳菌丝的存在极大地改变了发酵料中原有的微生物组成,导致T1和T2两个时期发酵料中微生物种群组成产生了显著差异。39种微生物在T2中显著富集,包括Cellulosimicrobium cellulans、Aminobacter J41、Ochrobactrum intermedium、Alcaligenes faecalis、Cellulosimicrobium funkei和Pseudomonas formosensis等(图2A)。T2和T3之间的差异分析表明,T3期较T2富集了更多的微生物(图2B)。一些属于纤维菌属、分枝杆菌属Mycobacterium和乔治菌属Georgenia的细菌在T3中显著富集,这些微生物能够产生多种降解酶如过氧化氢酶和漆酶,在有机质的降解中起重要作用(Wei et al. 2018 )。有趣的是,耐高温细菌如Thermobifida fusca和Thermobispora bispora在T2中富集,这可能是受到发酵料中高水平的氨气含量影响(Portilloet al. 2012 ;McGee et al. 2017 )。T3和T4之间,具有显著差异的微生物很少,只有糙皮侧耳和Alcaligenes faecalis在T4中表现出优势(图2C)。在这两个阶段,肉眼可见糙皮侧耳菌丝在发酵料中明显增加。据报道,Alcaligenes faecalis产生的代谢物具有抗真菌的作用,这有可能是发酵料栽培糙皮侧耳可以采用开放式接种的原因之一(El-Sayed et al. 2020 )。在T4和T5期菌落结构的比较中,T4和T5均富集了12种细菌(图2D)。总体来说,这些微生物在糙皮侧耳生长期间的富集可能对降解木质纤维素和抑制杂菌生长很重要。因此,发酵料中微生物组成变化将显著影响食用菌的生长和品质。
图2
图2
采用线性判别分析(LDA)效应量(LEfSe)方法对糙皮侧耳不同生长时期发酵料中的微生物在种水平上分类,LDA阈值为3.0
T1、T2、T3、T4和T5分别表示发酵完成(第0天,接种前)、菌丝定植(第3天)、发菌中期(第10天)、发菌末期(第20天,原体形成前)和P.ostreatus第一茬菇(第30天,子实体发育)
Fig. 2
Taxonomic differences at species level among bacteria in culture compost at the different growth stages of Pleurotus ostreatus using a linear discriminant analysis (LDA) effect size (LEfSe) method, and the LDA threshold is 3.0.
T1: Composting was completed (day 0, before spawning); T2: Mycelial colonization (day 3); T3: Mid-incubation (day 10); T4: End-incubation (day 20, before primordial formation); T5: First P.ostreatusfructification (day 30, fruiting body development).
2.3 糙皮侧耳不同生长时期发酵料中代谢组分的主成分分析
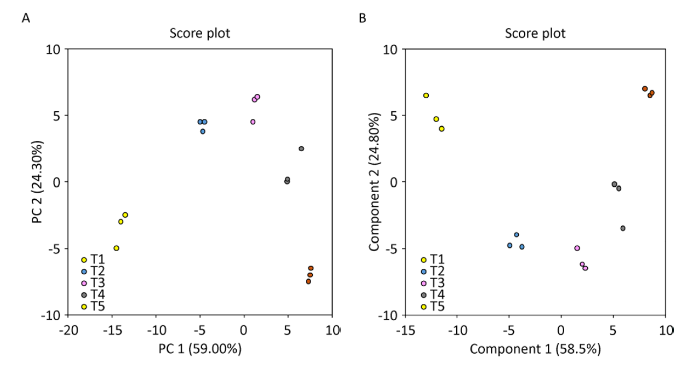
采用PCA法对糙皮侧耳T1、T2、T3、T4、T5这5个生长时期共15个发酵料样本代谢组分的数据进行统计分析。PCA得分结果显示,5组样本的PCA散点组间被很好地区分开,说明对5组样本数据处理是可信的,组间差异明显,组内样本有很好的集聚(图3A)。PCA是无监督的分析方法,不考虑数据的原始状态,直接观察实验样本的自然分布及组别关系,但该方法不能忽略组内误差,并消除随机误差(李艺筱 2016)。为进一步区分5组发酵料样本间代谢物的不同,确定发酵料中代谢组分在不同组间的差异成分,我们采用有监督的OPLS-DA对数据进行分析。从图3B上可进一步看出5组数据点在空间上呈现明显分离现象,表明5组发酵料中的代谢组分产生了显著差异。综上可知,在糙皮侧耳不同生长时期的发酵料中鉴定出的代谢物组间分离趋势均很好,这说明随着糙皮侧耳的生长发育,发酵料中的代谢物在种类和(或)含量上发生了显著变化。并且所有样本全部处于95%置信区间,模型无过度拟合现象且稳健性良好,可用于后续的差异成分分析。
图3
图3
糙皮侧耳不同生长时期发酵料中代谢物的PCA得分图(A)和OPLS-DA得分图(B)
T1、T2、T3、T4和T5分别表示发酵完成(第0天,接种前)、菌丝定植(第3天)、发菌中期(第10天)、发菌末期(第20天,原体形成前)和P.ostreatus第一茬菇(第30天,子实体发育)
Fig. 3
PCA (A) and OPLS-DA (B) scores of the metabolites in culture compost at the different growth stages of Pleurotus ostreatus.
T1: Composting was completed (day 0, before spawning); T2: Mycelial colonization (day 3); T3: Mid-incubation (day 10); T4: End-incubation (day 20, before primordial formation); T5: FirstP.ostreatusfructification (day 30, fruiting body development).
2.4 糙皮侧耳不同生长时期发酵料中差异代谢物分析
通过非靶向代谢组学技术对5组发酵料样本进行分析,共筛选和鉴定得到102个物质信号,其中差异代谢物有100个。这些差异代谢物可以归类为:氨基酸、核苷酸、糖类、有机酸、脂肪酸、维生素等(表1)。
表1 五组发酵料样本间的差异代谢物
Table 1
| 样品名称 Sample name | P值 P value | 出峰时间 Retention time (min) | 离子模式 Ion mode | 分子式 Formula | 质荷比 Precursor mass (Da) | 质量误差 Mass error |
|---|---|---|---|---|---|---|
| 胸腺嘧啶Thymine | 0.000704 | 4.7 | [M+H]- | C5H6N2O2 | 125.036 | 1 |
| 脱氧肌苷Deoxyinosine | 0.001465 | 4.7 | [M+H]- | C10H12N4O4 | 251.079 | -0.1 |
| 尿嘧啶Uracil | 7.01E-08 | 4 | [M+H]+ | C4H4N2O2 | 113.035 | 2.7 |
| 尿苷Uridine | 3.45E-07 | 4.1 | [M+H]- | C9H12N2O6 | 243.062 | 1.3 |
| 尿酸Urocanic acid | 2.92E-07 | 2 | [M+H]+ | C6H6N2O2 | 139.05 | -1 |
| 鸟嘌呤Guanine | 0.007398 | 1.6 | [M+H]+ | C5H5N5O | 152.057 | 0.2 |
| 鸟苷Guanosine | 2.98E-08 | 4.7 | [M+H]+ | C10H13N5O5 | 284.099 | -0.4 |
| 次黄嘌呤Hypoxanthine | 3.25E-08 | 2.7 | [M+H]+ | C5H4N4O | 137.046 | 0.4 |
| 胞苷Cytidine | 1.95E-07 | 1.6 | [M+H]+ | C9H13N3O5 | 244.093 | -1.2 |
| 胞苷一磷酸Cytidine monophosphate | 1.50E-08 | 2.1 | [M+H]+ | C9H14N3O8P | 324.059 | -1.3 |
| 肌苷Inosine | 7.14E-09 | 4.7 | [M+H]+ | C10H12N4O5 | 269.088 | -1.2 |
| 胞嘧啶Cytosine | 2.03E-07 | 1.6 | [M+H]+ | C4H5N3O | 112.051 | 3.9 |
| 脱氧胞苷Deoxycytidine | 1.14E-06 | 2.3 | [M+H]+ | C9H13N3O4 | 228.098 | -1 |
| 脱氧鸟苷Deoxyguanosine | 1.56E-09 | 4.7 | [M+H]+ | C10H13N5O4 | 268.104 | -0.9 |
| 1-甲基腺苷1-methyladenosine | 2.33E-06 | 3.2 | [M+H]+ | C11H15N5O4 | 282.12 | -0.5 |
| 3-甲基腺嘌呤3-methyladenine | 3.72E-11 | 1.6 | [M+H]+ | C6H7N5 | 150.077 | -2.4 |
| 7-甲基鸟嘌呤7-methylguanine | 1.74E-07 | 3 | [M+H]+ | C6H7N5O | 166.072 | -1.2 |
| 腺苷一磷酸Adenosine monophosphate | 2.16E-09 | 4.3 | [M+H]+ | C10H14N5O7P | 348.07 | 0.4 |
| 腺苷二磷酸ADP | 8.96E-08 | 1.6 | [M+H]+ | C10H15N5O10P2 | 428.037 | 0.4 |
| L-精氨酸L-arginine | 4.89E-07 | 0.9 | [M+H]+ | C6H14N4O2 | 175.119 | -0.9 |
| L-谷氨酸L-glutamic acid | 7.34E-05 | 1 | [M+H]+ | C5H9NO4 | 148.06 | 0 |
| L-谷氨酰胺L-glutamine | 1.77E-08 | 1 | [M+H]+ | C5H10N2O3 | 147.076 | -3.6 |
| L-组氨酸L-histidine | 2.15E-05 | 0.9 | [M+H]+ | C6H9N3O2 | 156.077 | -2.2 |
| L-异亮氨酸L-isoleucine | 1.25E-05 | 3.1 | [M+H]+ | C6H13NO2 | 132.102 | 2 |
| L-亮氨酸L-leucine | 4.15E-11 | 3.4 | [M+H]+ | C6H13NO2 | 132.102 | 1.5 |
| L-赖氨酸L-lysine | 2.65E-06 | 0.8 | [M+H]+ | C6H14N2O2 | 147.113 | -0.8 |
| L-蛋氨酸L-methionine | 2.51E-07 | 1.9 | [M+H]+ | C5H11NO2S | 150.058 | -1.4 |
| L-苯丙氨酸L-phenylalanine | 4.17E-05 | 4.7 | [M+H]+ | C9H11NO2 | 166.086 | -0.8 |
| L-天冬氨酸L-aspartic acid | 4.16E-05 | 1 | [M+H]- | C4H7NO4 | 132.03 | 3.6 |
| L-脯氨酸L-proline | 3.12E-07 | 1.1 | [M+H]+ | C5H9NO2 | 116.071 | 1.6 |
| L-苏氨酸L-threonine | 2.97E-08 | 1 | [M+H]+ | C4H9NO3 | 120.066 | 0.6 |
| L-色氨酸L-tryptophan | 3.97E-06 | 4.7 | [M+H]+ | C11H12N2O2 | 205.097 | -1.2 |
| L-酪氨酸L-tyrosine | 6.50E-07 | 3.4 | [M+H]+ | C9H11NO3 | 182.081 | -0.4 |
| L-缬氨酸L-valine | 3.88E-07 | 1.5 | [M+H]+ | C5H11NO2 | 118.086 | -0.2 |
| 瓜氨酸Citrulline | 3.99E-08 | 1 | [M+H]+ | C6H13N3O3 | 176.103 | -2 |
| 焦谷氨酸Pyroglutamic acid | 8.32E-08 | 2.7 | [M+H]+ | C5H7NO3 | 130.05 | 0.6 |
| 酮亮氨酸Ketoleucine | 1.50E-10 | 5.2 | [M+H]- | C6H10O3 | 129.056 | 4.5 |
| N-乙酰谷氨酸N-acetylglutamic acid | 9.17E-06 | 3.2 | [M+H]+ | C7H11NO5 | 190.071 | -2.2 |
| N-乙酰基-L-丙氨酸N-acetyl-L-alanine | 2.20E-07 | 3.7 | [M+H]- | C5H9NO3 | 130.051 | 4.7 |
| 甘氨酰脯氨酸Glycylproline | 5.73E-06 | 1.6 | [M+H]+ | C7H12N2O3 | 173.092 | -1.2 |
| 精氨琥珀酸Argininosuccinic acid | 4.27E-09 | 1 | [M+H]+ | C10H18N4O6 | 291.13 | -0.6 |
| 鞘氨醇Sphinganine | 3.29E-05 | 8.1 | [M+H]+ | C18H39NO2 | 302.305 | -0.1 |
| L-肉碱L-carnitine | 7.37E-10 | 1 | [M+H]+ | C7H15NO3 | 162.112 | -1.5 |
| 甘油磷酰胆碱Glycerophosphocholine | 2.06E-14 | 1 | [M+H]+ | C8H20NO6P | 258.11 | 0.1 |
| 甘油酸Glyceric acid | 1.51E-08 | 1.2 | [M+H]- | C3H6O4 | 105.019 | 4.2 |
| 甘油3-磷酸Glycerol 3-phosphate | 4.06E-10 | 1 | [M+H]- | C3H9O6P | 171.006 | -0.5 |
| 烟酰胺单核苷酸 Nicotinamide mononucleotide | 2.97E-08 | 1.4 | [M+H]+ | C11H15N2O8P | 335.064 | 0.1 |
| 烟酸Nicotinic acid | 0.000287 | 2 | [M+H]+ | C6H5NO2 | 124.039 | 0.9 |
| 6-羟基烟酸6-hydroxynicotinic acid | 0.000309 | 2.6 | [M+H]- | C6H5NO3 | 138.02 | 3.2 |
| 吡哆醇Pyridoxine | 6.67E-07 | 2.8 | [M+H]+ | C8H11NO3 | 170.081 | -1.7 |
| D-木糖D-xylose | 4.21E-11 | 1.6 | [M+H]- | C5H10O5 | 149.046 | 3.1 |
| 半乳糖醇Galactitol | 3.63E-13 | 1 | [M+H]- | C6H14O6 | 181.072 | 0.6 |
| 甘露醇Mannitol | 3.63E-13 | 1 | [M+H]- | C6H14O6 | 181.072 | 0.8 |
| 山梨糖醇Sorbitol | 3.63E-13 | 1 | [M+H]- | C6H14O6 | 181.072 | 0.6 |
| N-乙酰-D-氨基葡萄糖 N-acetyl-D-glucosamine | 7.35E-10 | 1.1 | [M+H]+ | C8H15NO6 | 222.097 | -1.1 |
| 尿苷二磷酸葡萄糖 Uridine diphosphate glucose | 1.29E-07 | 1.3 | [M+H]- | C15H24N2O17P2 | 565.048 | 1.4 |
| 2-异丙基苹果酸2-isopropylmalic acid | 1.30E-07 | 4.8 | [M+H]- | C7H12O5 | 175.061 | 1.8 |
| 3-甲基己二酸3-methyladipic acid | 3.28E-10 | 4.8 | [M+H]- | C7H12O4 | 159.066 | 2.7 |
| 4-羟基苯甲酸4-hydroxybenzoic acid | 9.91E-05 | 4.8 | [M+H]- | C7H6O3 | 137.024 | 4.6 |
| 4-丙酮酸4-pyridoxic acid | 4.00E-06 | 4.6 | [M+H]- | C8H9NO4 | 182.046 | -0.6 |
| 5-氨基戊酸5-aminopentanoic acid | 6.64E-05 | 1.6 | [M+H]- | C5H11NO2 | 116.072 | 4.5 |
| 壬二酸Azelaic acid | 5.69E-05 | 5.2 | [M+H]- | C9H16O4 | 187.098 | 1.8 |
| 柠苹酸Citramalic acid | 9.69E-06 | 3.4 | [M+H]- | C5H8O5 | 147.03 | 1.3 |
| 柠檬酸Citric acid | 1.74E-05 | 2.5 | [M+H]- | C6H8O7 | 191.02 | 2 |
| 葡萄糖酸Glucaric acid | 4.08E-08 | 1 | [M+H]- | C6H10O8 | 209.03 | 1 |
| D-核糖5-磷酸D-ribose 5-phosphate | 3.35E-06 | 1.1 | [M+H]- | C5H11O8P | 229.012 | 0.1 |
| 果糖6-磷酸Fructose 6-phosphate | 4.71E-09 | 1 | [M+H]- | C6H13O9P | 259.022 | 0.5 |
| 葡萄糖1-磷酸Glucose 1-phosphate | 4.71E-09 | 1 | [M+H]- | C6H13O9P | 259.022 | 0.5 |
| 葡萄糖6-磷酸Glucose 6-phosphate | 4.71E-09 | 1 | [M+H]- | C6H13O9P | 259.022 | 0.5 |
| 甘露糖6-磷酸Mannose 6-phosphate | 4.71E-09 | 1 | [M+H]- | C6H13O9P | 259.022 | 0.5 |
| 木酮糖5-磷酸Xylulose 5-phosphate | 9.74E-07 | 1.1 | [M+H]- | C5H11O8P | 229.012 | 0.1 |
| 十六烷基二酸Hexadecanedioic acid | 1.12E-07 | 9.5 | [M+H]- | C16H30O4 | 285.207 | 1.9 |
| 咪唑乙酸Imidazoleacetic acid | 1.51E-06 | 1.2 | [M+H]- | C5H6N2O2 | 125.036 | 4.2 |
| 4-胍基丁酸4-guanidinobutanoic acid | 1.02E-11 | 1.6 | [M+H]+ | C5H11N3O2 | 146.092 | -0.8 |
| 2-氨基异丁酸2-aminoisobutyric acid | 1.45E-05 | 1 | [M+H]+ | C4H9NO2 | 104.071 | 4 |
| N-甲基-α-氨基异丁酸 N-methyl-a-aminoisobutyric acid | 1.30E-08 | 1 | [M+H]+ | C5H11NO2 | 118.086 | 1.6 |
| 异柠檬酸Isocitric acid | 0.000147 | 1.3 | [M+H]- | C6H8O7 | 191.02 | 1 |
| 反式阿魏酸trans-ferulic acid | 1.63E-06 | 5.4 | [M+H]- | C10H10O4 | 193.051 | 1.8 |
| 异阿魏酸Isoferulic acid | 1.63E-06 | 5.4 | [M+H]- | C10H10O4 | 193.051 | 1.8 |
| L-苹果酸L-malic acid | 1.28E-09 | 1.5 | [M+H]- | C4H6O5 | 133.014 | 3.3 |
| 顺丁烯二酸Maleic acid | 1.99E-07 | 2.8 | [M+H]- | C4H4O4 | 115.004 | 3.9 |
| 中康酸Mesaconic acid | 1.73E-09 | 2.4 | [M+H]- | C5H6O4 | 129.019 | 2.9 |
| 甲基戊二酸Methylglutaric acid | 2.53E-07 | 4.8 | [M+H]- | C6H10O4 | 145.051 | 4.1 |
| 油酸Oleic acid | 0.000995 | 15 | [M+H]- | C18H34O2 | 281.249 | 1.1 |
| 酮戊二酸Oxoglutaric acid | 4.39E-09 | 1.6 | [M+H]- | C5H6O5 | 145.014 | 2.2 |
| 苯乙酮酸Phenylglyoxylic acid | 3.06E-09 | 4.8 | [M+H]- | C8H6O3 | 149.024 | 3.2 |
| 苯乳酸Phenyllactic acid | 0.000348 | 5.1 | [M+H]- | C9H10O3 | 165.056 | 1.6 |
| 邻苯二甲酸Phthalic acid | 2.42E-09 | 4.9 | [M+H]- | C8H6O4 | 165.019 | 2.2 |
| 喹啉酸Quinolinic acid | 0.000675 | 4.6 | [M+H]- | C7H5NO4 | 166.015 | 3.5 |
| 癸二酸Sebacic acid | 0.000566 | 5.5 | [M+H]- | C10H18O4 | 201.113 | 0.9 |
| 辛二酸Suberic acid | 5.73E-06 | 4.9 | [M+H]- | C8H14O4 | 173.082 | 0.4 |
| 琥珀酸Succinic acid | 4.11E-08 | 3.2 | [M+H]- | C4H6O4 | 117.019 | 4.7 |
| 创伤酸Traumatic acid | 0.000129 | 6.3 | [M+H]- | C12H20O4 | 227.129 | 1 |
| 十一碳二酸Undecanedioic acid | 1.67E-05 | 6 | [M+H]- | C11H20O4 | 215.129 | 1 |
| 4-羟基-3-甲基苯甲酸 4-hydroxy-3-methylbenzoic acid | 3.45E-11 | 5.5 | [M+H]+ | C8H8O3 | 153.055 | -1 |
| 2-氨基苯甲酸2-aminobenzoic acid | 3.46E-07 | 4.7 | [M+H]+ | C7H7NO2 | 138.055 | -1.9 |
| 吲哚-3-羧酸Indole-3-carboxylic acid | 0.000249 | 5.3 | [M+H]- | C9H7NO2 | 160.04 | 3.6 |
| 吲哚丙烯酸Indoleacrylic acid | 5.90E-12 | 4.7 | [M+H]+ | C11H9NO2 | 188.071 | -0.9 |
| 吲哚Indole | 9.17E-08 | 5.4 | [M+H]+ | C8H7N | 118.065 | 0 |
| 葫芦巴碱Trigonelline | 1.04E-08 | 1.1 | [M+H]+ | C7H7NO2 | 138.055 | -0.9 |
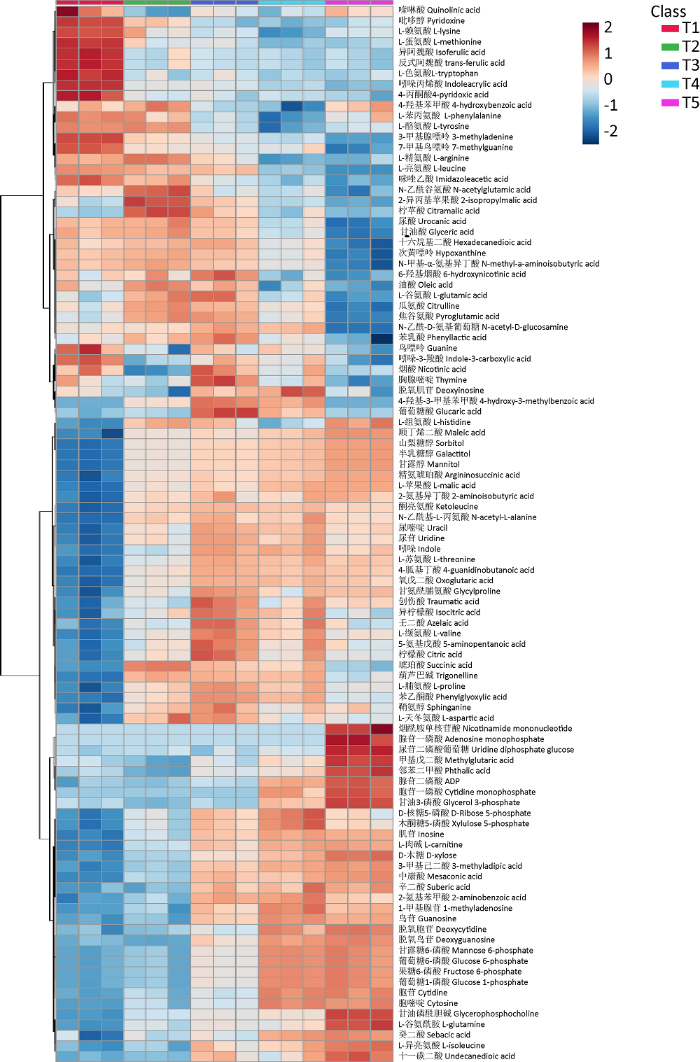
图4
图4
糙皮侧耳不同生长时期发酵料中代谢物的层次聚类分析热力图
T1、T2、T3、T4和T5分别表示发酵完成(第0天,接种前)、菌丝定植(第3天)、发菌中期(第10天)、发菌末期(第20天,原体形成前)和P.ostreatus第一茬菇(第30天,子实体发育)
Fig. 4
Heatmap of hierarchical clustering analysis for the metabolites in culture compost at the different growth stages of Pleurotus ostreatus.
T1: Composting was completed (day 0, before spawning); T2: Mycelial colonization (day 3); T3: Mid-incubation (day 10); T4: End-incubation (day 20, before primordial formation); T5: First P.ostreatusfructification (day 30, fruiting body development).
发酵料制备完成期(T1)检测到较高含量的氨基酸类、维生素类和阿魏酸类物质。微生物在发酵料制备过程中起着重要的作用,一方面可以将大分子物质如蛋白质、木质素降解成小分子物质如氨基酸、阿魏酸等(王丽 2014),另一方面可以进行营养转化,合成维生素等物质,为糙皮侧耳生长提供营养(Zhouet al. 2019 )。此外3-甲基腺嘌呤、7-甲基鸟嘌呤、鸟嘌呤、胸腺嘧啶在T1时期含量也较高,此类物质在细胞的内外分泌、功能的调节以及微生物生长、增殖过程中都具有重要的影响(雷露等 2020),这说明此时发酵料中的微生物核酸代谢旺盛,菌体活性高。
三羧酸(TCA)循环是生物体获得能量的最有效方式,亦是体内三大营养素(糖类、脂类、氨基酸)联系的枢纽。此外,TCA循环所产生的多种中间产物是体内许多重要物质生物合成的原料。在细胞迅速生长时期,TCA循环可提供多种化合物的碳架和能量,以供细胞生物合成使用(谈梦飞等 2018;雷露等 2020)。在菌丝生长时期(T2-T4),TCA循环的中间产物如柠檬酸、异柠檬酸、琥珀酸、氧戊二酸含量较高,说明在此期间TCA循环较强,能够为糙皮侧耳菌丝快速生长提供重要物质合成所需的碳架和较多的能量。在食用菌生长过程中,菌丝能够通过相关酶将蛋白质分解成氨基酸和肽类物质,满足其自身和子实体快速生长所需,并赋予糙皮侧耳丰富营养和鲜香口感(Aguirre et al. 2008 )。检测到的氨基酸类和肽类物质如L-组氨酸、L-苏氨酸、L-甘氨酰脯氨酸、L-缬氨酸、L-脯氨酸、L-天冬氨酸、谷氨酰胺、酮亮氨酸、L-异亮氨酸在菌丝生长和子实体发育期(T2-T5)含量较高。其中,天冬氨酸是生物体内相关氨基酸类物质、4种碱基合成的前体物质或蛋白质从头合成所需α-氨基和氨氮的碳受体,并可维持机体的氮平衡(王秋菊等 2011;王波 2020)。此外,天冬氨酸还可以影响具有调节糖类物质、蛋白质、脂肪酸代谢作用的重要因子辅酶A。谷氨酰胺可以被磷酸激活的谷氨酰胺酶水解为谷氨酸,进一步可以合成还原性谷胱甘肽,参与机体抗氧化状态动态平衡的维持(王秋菊等 2011)。此外,天冬氨酸和谷氨酸还是食用菌体内味精样(monosodium glutamate-like,MSG-like)物质,有助于食用菌特征风味的形成(Mau 2001)。在生物体内,苏氨酸可以不经过脱氨基和转氨基作用,直接在苏氨酸脱水酶、苏氨酸脱氢酶和苏氨酸醛缩酶的催化下转变为丁酰辅酶A、琥珀酰辅酶A、丝氨酸、甘氨酸等物质(王雅梅和管丹彤 2012;张丽媛等 2020)。多糖是一种具有多种生物活性的物质。在相关酶的作用下,糙皮侧耳可以利用碳源(葡萄糖)合成由甘露糖、葡萄糖、半乳糖等通过糖苷键聚合而成的糙皮侧耳菌丝多糖(Komuraet al. 2014 )。由图4可知,木糖和一些单糖的衍生物如山梨糖醇、半乳糖醇、甘露糖醇随着糙皮侧耳生长,含量不断增加。山梨糖醇是碳水化合物的运输形式之一,也是一种可溶性的贮藏碳水化合物。在多元醇途径中,葡萄糖经醛糖还原酶催化还原成山梨糖醇,后者在山梨糖醇脱氢酶的作用下转变为果糖。半乳糖醇、山梨糖醇可能是由葡萄糖或甘露糖经醛糖还原酶催化还原而成(张丽媛等 2020)。
食用菌营养生长后期(T4)和生殖生长期(T5),菌丝需要提供大量的营养和能量,以利于原基形成和子实体发育。因此发酵料中参与糖类代谢、脂肪代谢及能量代谢的物质如烟酰胺单核苷酸、腺苷一磷酸、尿苷二磷酸葡萄糖、ADP、胞苷一磷酸、甘油3-磷酸、D-核糖5-磷酸、木酮糖5-磷酸、甘露糖6-磷酸、葡萄糖6-磷酸、果糖6-磷酸、葡萄糖1-磷酸、胞苷、胞嘧啶、甘油磷酸胆碱、肉碱等在此时期含量较高。
2.5 差异代谢物的代谢途径分析
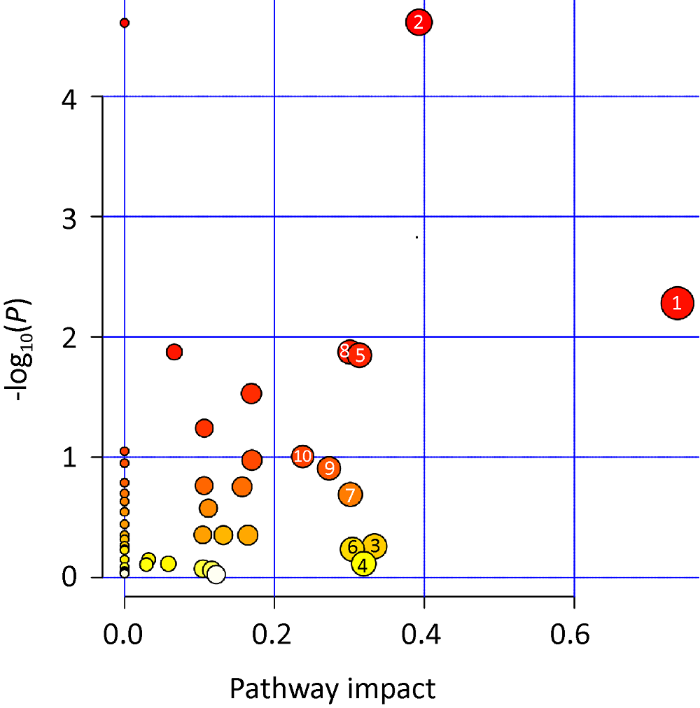
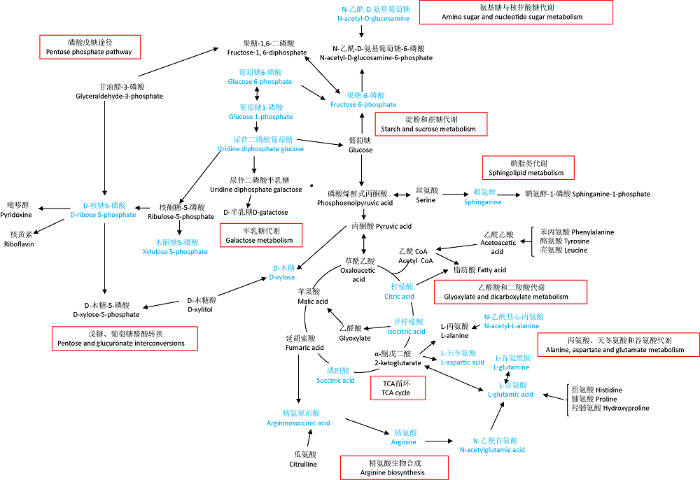
Metaboanalyst 4.0是一个基于网络的定量代谢组数据综合分析平台,可以通过拓扑分析和功能数据分析将差异代谢物进行分配并得出重要的代谢途径(Chong et al. 2019 ;雷露等 2020)。KEGG通路富集分析表明,100个差异代谢物共富集到48条代谢途径(图5)。其中影响值(pathway impact)大于0.2的关键代谢途径有10条,参与其中的差异代谢物共19种。分别为:(1)丙氨酸、天冬氨酸和谷氨酸代谢(alanine,aspartate and glutamate metabolism)、(2)精氨酸生物合成(arginine biosynthesis)、(3)半乳糖代谢(galactose metabolism)、(4)鞘脂类代谢(sphingolipid metabolism)、(5)乙醛酸和二羧酸代谢(glyoxylate and dicarboxylatemetabolism)、(6)磷酸戊糖途径(pentose phosphate pathway)、(7)淀粉和蔗糖代谢(starch and sucrose metabolism)、(8)TCA循环(TCA cycle)、(9)戊糖和葡萄糖醛酸的相互转化(pentose and glucuronate interconversions)、(10)氨基糖与核苷酸糖代谢(amino sugar and nucleotide sugar metabolism)。相关差异代谢物与关键途径见图6,图中蓝色表示差异代谢物,黑色表示为代谢途径中的中间产物。
图5
图5
差异代谢通路富集分析
Pathway impact表示差异代谢物在该途径的富集程度影响值,气泡大小表示差异代谢物富集数目多少,颜色深浅表示-log10(P)值大小
Fig. 5
Differential metabolite pathway enrichment analysis.
Pathway impact indicates the enrichment degree impact of differential metabolites; the bubble indicates the number of enrichment of differential metabolites; the color indicates the value of -log10(P) value.
图6
图6
差异代谢物代谢途径分析
Fig. 6
Metabolic pathway analysis of the differential metabolites.
2.6 糙皮侧耳-微生物-代谢物相关性分析
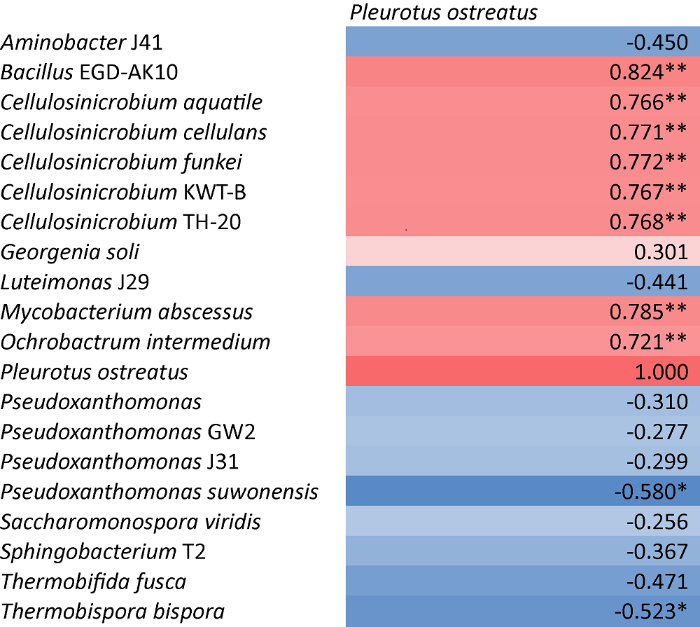
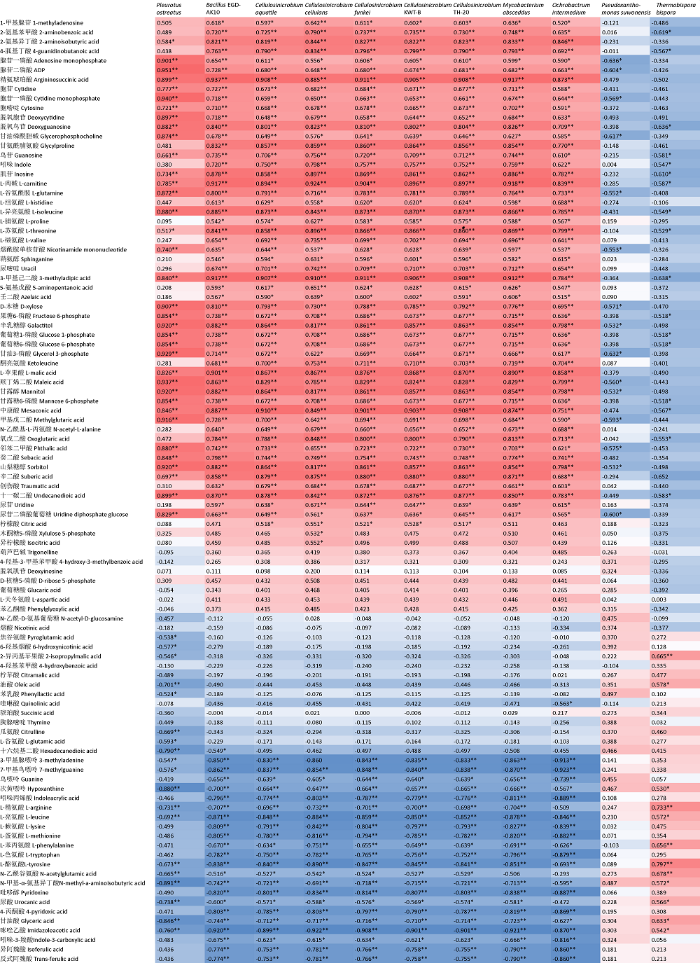
发酵料中的细菌如Pseudoxanthomonas suwonensis和Thermobispora bispora与糙皮侧耳的相对丰度呈显著负相关关系(P<0.05)(图7)。与糙皮侧耳相对丰度呈负相关关系的代谢物如2-异丙基苹果酸、油酸、次黄嘌呤、精氨酸、亮氨酸、酪氨酸、N-乙酰谷氨酸、N-甲基-α-氨基异丁酸、尿酸、甘油、咪唑乙酸的含量与Thermobispora bispora的相对丰度呈显著正相关关系(图8)。反之,BacillusEGD-AK10、Cellulosinicrobium aquatile、Cellulosinicrobium cellulans、Cellulosinicrobium funkei、CellulosinicrobiumKWT-B、CellulosinicrobiumTH-20、Mycobacterium abscessus、Ochrobactrum intermedium 8种微生物与糙皮侧耳的相对丰度呈显著正相关关系(P<0.01)。并且与糙皮侧耳相对丰度呈显著正相关的代谢物如精氨酸琥珀酸、胞苷、胞嘧啶、脱氧胞苷、脱氧鸟苷、ADP、甘油磷脂酰胆碱、D-木糖、果糖6-磷酸、半乳糖醇、葡萄糖1-磷酸、葡萄糖6-磷酸、甘油3-磷酸、甘露糖6-磷酸、甘露醇、山梨糖醇、烟酰胺单核苷酸、苏氨酸等的含量与上述8种微生物的相对丰度亦呈显著正相关关系(P<0.05)。说明这些微生物和代谢物对糙皮侧耳的生长发育具有重要作用,这为研究糙皮侧耳生长发育过程中,发酵料中微生物和代谢的变化提供了重要的信息。由此推测,发酵料中的芽孢杆菌属、纤维菌属的一些特定细菌相对丰度的升高会引起苏氨酸、组氨酸、葡萄糖6-磷酸、D-木糖、甘露醇等代谢物含量的增加,从而促进糙皮侧耳的生长发育。
图7
图7
糙皮侧耳和发酵料中相对丰度前20的物种之间的Pearson相关系数
*P<0.05,**P<0.01
Fig. 7
Pearson correlation coefficients between Pleurotus ostreatus and the 20 dominant species in culture compost.
*P<0.05, **P<0.01.
图8
图8
代谢物含量与特定物种相对丰度之间的Pearson相关系数
*P<0.05,**P<0.01
Fig. 8
Pearson correlation coefficients between the metabolite content and the relative abundance of specific species.
*P<0.05, **P<0.01.
3 讨论
食用菌的种植包括为食用菌生长和发育提供支撑物和营养的土壤或选择性培养基(Carrasco & Preston 2020)。存在于种植环境中的微生物对蘑菇的生长和发育具有显著的影响,甚至在某些情况下是必须的。据报道,芽孢杆菌属、固氮菌属Azotobacter、类芽孢杆菌属Paenibacillus和假单胞菌属Pseudomonas中的一些特定微生物,可以:(1)将木质纤维素生物质降解为简单、易于为食用菌吸收利用的物质;(2)促进食用菌菌丝生长,诱导子实体形成;(3)对竞争性霉菌表现出拮抗作用(Carrasco et al. 2018 ;Kertesz & Thai 2018)。另一方面,食用菌菌丝的存在会改变培养料中原有的微生物群落的组成和多样性。在双孢蘑菇发菌过程中,双孢蘑菇与变形菌门成员之间存在一定的协同作用,双孢蘑菇的存在导致变形菌门,尤其是假单胞菌属相对丰度的增加,而厚壁菌门和放线菌门Actinobacteria的相对丰度显著降低(Carrasco & Preston 2020)。本研究中,随着糙皮侧耳的生长发育,发酵料中的BacillusEGD-AK10、Cellulosinicrobium aquatile、Cellulosinicrobium cellulans、Cellulosinicrobium funkei、CellulosinicrobiumKWT-B、Cellulosinicrobium TH-20、Mycobacterium abscessus、Ochrobactrum intermedium的相对丰度明显增加,并与糙皮侧耳的相对丰度呈显著正相关关系,而Pseudoxanthomonas suwonensis和Thermobispora bispora与糙皮侧耳的相对丰度呈显著负相关关系。这种微生物群落结构的变化可能是由于糙皮侧耳生长过程中,发酵料中形成了一个特定的、利于糙皮侧耳生长的微生物种群,其独特地适应了糙皮侧耳和发酵料中的木质纤维素生物质。糙皮侧耳不同生长阶段,发酵料中的微生物发生了显著的变化,而不同的微生物必然产生不同的代谢物。从这个意义上讲,发酵料中存在的微生物参与了发酵料的代谢,从而影响了糙皮侧耳的生长。采用UPLC-QTOF-MS结合多变量统计方法对糙皮侧耳5个典型生长时期的发酵料样品中的代谢物的变化进行分析,共检测到102种代谢物,其中差异代谢物共有100种,主要集中于氨基酸合成代谢和TCA循环通路。通过PCA和OPLS-DA分析可知5组样品之间的代谢物具有明显差异。其中与糙皮侧耳相对丰度呈显著正相关关系的代谢物有36种,呈显著负相关关系的代谢物有19种,主要包括糖类、脂类、氨基酸、核苷酸等。并且与糙皮侧耳相对丰度具有显著正相关关系的8种微生物的相对丰度和上述36种代谢物含量之间亦呈显著正相关关系,这意味着BacillusEGD-AK10、Cellulosinicrobium aquatile、Cellulosinicrobium cellulans等相对丰度的升高,引起L-组氨酸、L-苏氨酸、葡萄糖6-磷酸、D-木糖、甘露醇等代谢物含量的增多,促进糙皮侧耳的生长发育。
本研究虽然发现了糖类、脂类、氨基酸和核苷酸等一系列参与糙皮侧耳生长发育的物质,探明了它们随糙皮侧耳生长发育的变化规律,但是这些代谢物与微生物、糙皮侧耳间的关系尚不明确。发酵料中的微生物间既竞争又合作,关系错综复杂;细胞的代谢途径间亦是相互衔接影响、协调响应的,如氮代谢和碳代谢是相互衔接的,属于碳代谢的糖酵解途径和TCA循环可以产生用于氨基酸合成所需的碳骨架,而普遍认为氨基酸代谢属于氮代谢;丝氨酸可以与甘油磷脂生成磷脂酰丝氨酸,参与脂质代谢;脂质分解产物之一的丙酮酸进入TCA循环,参与碳代谢;碳代谢中产生的碳骨架再次形成氨基酸;α-L-氨基酸可以衍生为B族维生素,以维生素为前体生成的代谢物可以进一步参与碳代谢,此外氨基酸分解所生成的α-酮酸可以转变成糖、脂类或再合成某些非必需氨基酸,也可以经过TCA循环氧化成二氧化碳和水,并放出能量,这些代谢过程相互影响(闫志宇 2020)。代谢物在特定的情况下的改变是协调变化的,代谢产物的变化与微生物体内相关酶活性息息相关,而生物体内的大多数酶活力变化亦是精密调控的,比如参与TCA循环的酶(Sulpice et al. 2010 )。因此需要进一步综合分析发酵料中的微生物和代谢物种类、含量的关系。
本研究表明,糙皮侧耳生长过程中发酵料中的微生物和代谢物有着显著的改变,三者之间存在精密复杂、相互协调的多层次响应关系。这提示我们应该从更广阔的角度探索糙皮侧耳-微生物-代谢物之间的关系,如不同代谢途径的互作、不同响应层次间的联系等。本研究对糙皮侧耳促生长策略的制定,为提高食用菌商业化栽培效率提供了一定的理论基础。
参考文献
Modification in the properties of paper by using cellulase-free xylanase in biobleaching of wheat straw pulp produced from alkalophilic Cellulosimicrobium cellulans CKMX1
DOI:10.1021/ci400128m URL [本文引用: 1]
Enzymatic hydrolysis of soybean protein using lactic acid bacteria
DOI:10.1016/j.foodchem.2008.05.018 URL [本文引用: 1]
Biodegradation of bispyribac sodium by a novel bacterial consortium BDAM: optimization of degradation conditions using response surface methodology
DOI:10.1016/j.jhazmat.2017.12.065 URL [本文引用: 1]
Sampling for metabolome analysis of microorganisms
DOI:10.1021/ac0623888 URL [本文引用: 1]
Holistic assessment of the microbiome dynamics in the substrates used for commercial champignon (Agaricus bisporus) cultivation
DOI:10.1111/mbt2.v13.6 URL [本文引用: 1]
Growing edible mushrooms: a conversation between bacteria and fungi
DOI:10.1111/emi.v22.3 URL [本文引用: 6]
Supplementation in mushroom crops and its impact on yield and quality
DOI:10.1186/s13568-018-0678-0
PMID:30229415
[本文引用: 1]

Mushroom supplementation is an agronomic process which consists of the application of nutritional amendments to the substrates employed for mushroom cultivation. Different nitrogen and carbohydrate rich supplements have been evaluated in crops with a substantial impact on mushroom yield and quality; however, there is still controversy regarding the nutritional requirements of mushrooms and the necessity for the development of new commercial additives. The addition of external nutrients increases the productivity of some low-yielding mushroom varieties, and therefore is a useful tool for the industry to introduce new commercially viable varieties. Spent mushroom compost is a waste material that could feasibly be recycled as a substrate to support a new commercially viable crop cycle when amended with supplements. On the other hand, a new line of research based on the use of mushroom growth promoting microorganisms is rising above the horizon to supplement the native microbiota, which appears to cover nutritional deficiencies. Several supplements employed for the cultivated mushrooms and their agronomic potential in terms of yield and quality are reviewed in this paper as a useful guide to evaluate the nutritional requirements of the crop and to design new formulas for commercial supplementation.
Properties of the newly isolated extracellular thermo-alkali-stable laccase from thermophilic actinomycetes, Thermobifida fusca and its application in dye intermediates oxidation
DOI:10.1186/2191-0855-3-49 URL [本文引用: 1]
MetaboAnalystR 2.0: from raw spectra to biological insights
DOI:10.3390/metabo9030057 URL [本文引用: 1]
A revised checklist of medicinal fungi in China
A revised checklist of edible fungi in China
Octadecyl 3-(3, 5-di-tert- butyl-4-hydroxyphenyl) propanoate, an antifungal metabolite of Alcaligenes faecalisstrain MT332429 optimized through response surface methodology
DOI:10.1007/s00253-020-10962-9 URL [本文引用: 1]
Metabolomics - the link between genotypes and phenotypes
DOI:10.1023/A:1013713905833 URL [本文引用: 1]
Metabolomic differences among different parts of Isaria cicadae cultured on Antheraea pernyi
A simple procedure for preparing substrate for Pleurotus ostreatus cultivation
DOI:10.1016/S0960-8524(03)00118-4 URL [本文引用: 2]
Pleurotus ostreatus, an oyster mushroom, decreases the oxidative stress induced by carbon tetrachloride in rat kidneys, heart and brain
DOI:10.1016/j.cbi.2008.08.006 URL [本文引用: 1]
Compost bacteria and fungi that influence growth and development of Agaricus bisporus and other commercial mushrooms
DOI:10.1007/s00253-018-8777-z URL [本文引用: 1]
Water-soluble polysaccharides from Pleurotus ostreatus var. florida mycelial biomass
DOI:10.1016/j.ijbiomac.2014.06.007 URL [本文引用: 1]
Metagenomic analysis revealed the succession of microbiota and metabolic function in corncob composting for preparation of cultivation medium for Pleurotus ostreatus
Analysis of metabolic differences in fermentation of Grifola frondosa based on UPLC-QTOF-MS metabolomics
Study on the pathway by metabolomics of yellowing substrate from fresh-cut Chinese water chestnut (Eleocharis tuberosa) and the related enzymes
Distinct changes of characteristic compounds in garlic during growth and storage based on metabolomics
The umami taste of edible and medicinal mushrooms
DOI:10.1615/IntJMedMushr.v7.i12 URL [本文引用: 1]
Diversity and dynamics of the DNA and cDNA-derived bacterial compost communities throughout theAgaricus bisporus mushroom cropping process
Production of bio-fertilizer from microwave vacuum pyrolysis of palm kernel shell for cultivation of oyster mushroom (Pleurotus ostreatus)
DOI:10.1016/j.scitotenv.2017.12.108 URL [本文引用: 1]
Presence and potential role of thermophilic bacteria in temperate terrestrial environments
DOI:10.1007/s00114-011-0867-z
PMID:22159635
[本文引用: 1]

Organic sulfur and nitrogen are major reservoirs of these elements in terrestrial systems, although their cycling remains to be fully understood. Both sulfur and nitrogen mineralization are directly related to microbial metabolism. Mesophiles and thermophiles were isolated from temperate environments. Thermophilic isolates were classified within the Firmicutes, belonging to the Geobacillus, Brevibacillus, and Ureibacillus genera, and showed optimum growth temperatures between 50°C and 60°C. Sulfate and ammonium produced were higher during growth of thermophiles both for isolated strains and natural bacterial assemblages. They were positively related to organic nutrient load. Temperature also affected the release of sulfate and ammonium by thermophiles. Quantitative, real-time reverse-transcription polymerase chain reaction on environmental samples indicated that the examined thermophilic Firmicutes represented up to 3.4% of the total bacterial community RNA. Temperature measurements during summer days showed values above 40°C for more than 10 h a day in soils from southern Spain. These results support a potential role of thermophilic bacteria in temperate terrestrial environments by mineralizing organic sulfur and nitrogen ruled by the existence and length of warm periods.
Cultivation of Pleurotus ostreatusand other edible mushrooms
DOI:10.1007/s00253-009-2343-7 URL [本文引用: 1]
At the crossroads of bacterial metabolism and virulence factor synthesis in staphylococci
DOI:10.1128/MMBR.00005-09
PMID:19487727
[本文引用: 1]

Bacteria live in environments that are subject to rapid changes in the availability of the nutrients that are necessary to provide energy and biosynthetic intermediates for the synthesis of macromolecules. Consequently, bacterial survival depends on the ability of bacteria to regulate the expression of genes coding for enzymes required for growth in the altered environment. In pathogenic bacteria, adaptation to an altered environment often includes activating the transcription of virulence genes; hence, many virulence genes are regulated by environmental and nutritional signals. Consistent with this observation, the regulation of most, if not all, virulence determinants in staphylococci is mediated by environmental and nutritional signals. Some of these external signals can be directly transduced into a regulatory response by two-component regulators such as SrrAB; however, other external signals require transduction into intracellular signals. Many of the external environmental and nutritional signals that regulate virulence determinant expression can also alter bacterial metabolic status (e.g., iron limitation). Altering the metabolic status results in the transduction of external signals into intracellular metabolic signals that can be "sensed" by regulatory proteins (e.g., CodY, Rex, and GlnR). This review uses information derived primarily using Bacillus subtilis and Escherichia coli to articulate how gram-positive pathogens, with emphasis on Staphylococcus aureus and Staphylococcus epidermidis, regulate virulence determinant expression in response to a changing environment.
Network analysis of enzyme activities and metabolite levels and their relationship to biomass in a large panel of Arabidopsis accessions
DOI:10.1105/tpc.110.076653
PMID:20699391
[本文引用: 1]

Natural genetic diversity provides a powerful resource to investigate how networks respond to multiple simultaneous changes. In this work, we profile maximum catalytic activities of 37 enzymes from central metabolism and generate a matrix to investigate species-wide connectivity between metabolites, enzymes, and biomass. Most enzyme activities change in a highly coordinated manner, especially those in the Calvin-Benson cycle. Metabolites show coordinated changes in defined sectors of metabolism. Little connectivity was observed between maximum enzyme activities and metabolites, even after applying multivariate analysis methods. Measurements of posttranscriptional regulation will be required to relate these two functional levels. Individual enzyme activities correlate only weakly with biomass. However, when they are used to estimate protein abundances, and the latter are summed and expressed as a fraction of total protein, a significant positive correlation to biomass is observed. The correlation is additive to that obtained between starch and biomass. Thus, biomass is predicted by two independent integrative metabolic biomarkers: preferential investment in photosynthetic machinery and optimization of carbon use.
Metabolomics analysis of Tween-60 on pullulan by Aureobasidium pullulans
Microbial biomass in compost during colonization of Agaricus bisporus
DOI:10.1186/s13568-016-0304-y URL [本文引用: 1]
Physiological change and analysis of differential metabolites of mother bulb during the growing period of Lilium davidii var. unicolor
Purification and characterization of feruloyl esterases from Panus giganteus,Russula virescens andLactarius hatsudake
Nitrogen transformation processes and the diversity of functional microbial communities in polar environment
Recent advances in transport systems of glutamate and glutamine
Relationship between threonine nutrition and immunity of livestock and poultry
Succession of the functional microbial communities and the metabolic functions in maize straw composting process
DOI:10.1016/j.biortech.2018.02.050 URL [本文引用: 1]
Effect of inoculating microbes in municipal solid waste composting on characteristics of humic acid
DOI:10.1016/j.chemosphere.2006.12.067 URL [本文引用: 1]
Resource diversity of Chinese macrofungi: edible, medicinal and poisonous species
DOI:10.1007/s13225-019-00432-7 URL [本文引用: 1]
Mapping the metabolic signatures of fermentation broth, mycelium, fruiting body and spores powder from Ganoderma lucidumby untargeted metabolomics
DOI:10.1016/j.lwt.2020.109494 URL [本文引用: 1]
Core carbon metabolism response of Pleurotus ostreatus to high temperature stress
Separation and identification of metabolites and metabolic mechanism analysis in two kinds of mung bean
Changes in plasma metabolites after calving in dairy cows
Dynamic changes and functional analysis of bacteria during the fermentation of Da-jiang
Succession of organics metabolic function of bacterial community in response to addition of earthworm casts and zeolite in maize straw composting
DOI:10.1016/j.biortech.2019.02.015 URL [本文引用: 2]
中国药用真菌名录及部分名称的修订
中国食用菌名录
极地环境氮转化及其功能微生物群落多样性
不同品种绿豆中代谢产物的分离鉴定及代谢机制分析