马蒂菌属Mattirolomyces E. Fisch.由Fischer 在1938年建立,以地菇状马蒂菌Mattirolomyces terfezioides (Mattir.) E. Fisch.作为模式种。随后美国学者将该物种归到“沙漠块菌”地菇属Terfezia (俗称沙漠块菌) (Trappe 1971),而将马蒂菌属降级为地菇属亚属。Percudani et al. (1999)基于18S rDNA的分子系统学分析重新将网孢地菇Terfezia terfezioides (≡Mattirolomyces terfezioides)归到单系起源的Mattirolomyces属中,确立了其在子囊菌门盘菌科中的分类地位。随后,Norman & Egger (1999)的研究也支持了这一结论。
马蒂菌属Mattirolomyces曾经以单种M. terfezioides的形式存在近一个世纪。目前,该属包含已报道的6个种即欧洲的M. terfezioides (Mattir.) E. Fisch、南非的M. austroafricanus (Marasas & Trappe) Kovács, Trappe & Claridge、墨西哥的M. mexicanus Kovács, Trappe & Alsheikh、澳大利亚的M. mulpu Kovács, Trappe & Claridge以及北美的M. spinosus (Harkn.) Kovács, Trappe & Alsheikh和M. tiffanyae Healy (Trappe et al. 2010a,2010b;Kovács et al. 2011b),主要生长在欧洲中部和南部的温带森林或刺槐人工林Robinia pseudoacacia、柿树Diospyros kaki和桃树Prunus avium下,区别于Terfezia属的类群主要分布在地中海地区、中东及亚洲西南地区的沙漠中(Kovács et al. 2011a)。Kovács et al. (2007)研究Mattirolomyces及其近缘属分类群的菌根时没有发现M. terfezioides有典型的菌根特征,但可以形成菌核“sclerotia”,而且在其宿主植物刺槐R. pseudoacacia根皮层细胞中发现有隔菌丝圈,推测其与M. terfezioides 的菌丝结构相似,暗示其可能是内生真菌。
已有文献记录大型地下真菌地菇状马蒂菌M. terfezioides (≡Terfezia terfezioides) (又名网孢地菇)在我国北京、河北、山西以及河南等地有分布(刘波等 2002),并且与该属另外两个种:刺孢地菇Terfezia spinosa Harkn和瘤孢地菇Terfezia arenaria (Moris) Trappe,同列在我国可食用的大型真菌名录中(戴玉成等 2010;Wu et al. 2019)。Wang et al. (2017)基于馆藏干标本描述了M. terfezioides的形态特征并提供了DNA分子数据,为M. terfezioides在我国的分布提供了新的补充资料。笔者在最近一次地下真菌的野外调查过程中,在我国河北省保定市杨园桃树下发现白色假块菌,经鉴定为M. terfezioides,现将其新鲜子囊果特征及生态学特征做一详细描述。由于当地居民将其作为“白块菌”采食,为更好地理解该物种特征,我们同时检测了新鲜子囊果的挥发性有机化合物(VOCs)的组成和含量,并与新鲜块菌属Tuber成员进行了比较分析和讨论。
1 材料与方法
研究材料分别于2020年8、9月及10月3次采自河北省保定市满城县杨园农家桃园内。凭证标本均存于中国医学科学院药用植物研究所真菌室。
1.1 形态学观察
原地采集新鲜样品时观察子囊果的外观特征和质地、产孢组织的质地和颜色等宏观特征;生境为农家桃园,以桃树为主,周边有柿树。样品采集时小心将子囊果及植物根样放于自封袋内,当天低温运回实验室于4 ℃冰箱中保藏。随后在实验室内进行子囊果的微观形态学观察,包括子囊果表面、包被的组成、子囊的形状、大小和所含的孢子数目;孢子的形状、大小和表面纹饰等。
1.2 DNA提取、测序及序列分析
取新鲜样品的子囊果100 mg,用液氮研磨成粉末状,采用十六烷基三甲基溴化铵(cetyltrimethylammonium bromide,CTAB)植物基因组DNA快速提取试剂盒(购自北京艾德莱生物科技有限公司)进行样品的DNA提取。DNA提取及PCR扩增参照试剂盒说明书及李佳梅等(2019)的方法。PCR扩增采用真菌特异性引物ITS1F/ITS4 (Gardes & Bruns 1993)扩增核糖体内转间隔区(internal transcribed space,ITS)。PCR产物经电泳检测后送金唯智(北京)生物技术有限公司进行一代测序。利用MEGA Version 7.0软件(www.megasoftware.net)对序列进行排序并用邻接法(neighbor-joining method)进行系统发育树的构建。
1.3 挥发性有机化合物的检测
釆用顶空固相微萃取-气相色谱-质谱联用技术(SPME-GC-MS)测定新鲜地菇状马蒂菌子囊果挥发性有机化合物的成分及含量。取5.0 g样品于20 mL顶空瓶中,60 ℃下平衡5 min后,用固相微萃取针萃取40 min,使用前将萃取针在250 ℃下老化10 min。
气相条件:采用不分流进样,进样温度为250 ℃,色谱柱为HP-5MS毛细管柱(30 m×0.25 mm× 0.25 μm)。升温程序为:起始温度为50 ℃,保持2 min,以5 ℃/min升至180 ℃并保持5 min,再以10 ℃/min升至250 ℃并保持5 min。载气为He,流速1.2 mL/min,分离比5:1。质谱条件:EI离子源电压为70 eV,离子源温度为230 ℃,四极杆温度为150 ℃,传输线温度为280 ℃,扫描范围为30-500 m/z。
色谱分离后,质谱扫描每一个色谱峰得到质谱图,经计算机自动匹配标准NIST (National Institute of Standards and Technology)质谱库,再根据匹配度较高的结果以及相关文献标准质谱图核对分析,确定样品中挥发性化合物的种类。釆用面积归一法计算出每一个样品中挥发性成分的相对百分含量,确定该子囊果中挥发性物质的组成与含量(3个生物学重复)。
2 结果与分析
2.1 地菇状马蒂菌的形态学特征
Mattirolomyces terfezioides (Mattir.) E. Fisch. in Fischer in Engler & Prantl, Nat. Pflanzenfam., Edn. 2 (Leipzig) 5b(7): 39, 1938
≡Choiromyces terfezioides Mattir. Mém. R. Accad. Sci. Torino, Ser. 2 37: 10, 1887
≡Terfezia terfezioides (Mattir.) Trappe, Trans. Br. Mycol. Soc. 57(1): 91, 1971
图1
图1
地菇状马蒂菌的形态特征
A-C:新鲜子囊果特征;D:包被及产孢组织;E,F:子囊及球状带刺状纹饰子囊孢子;G:宿主植物根部土块内包裹的幼嫩子囊果;H:幼根尖膨大成棒状似外生菌根结构;I:根横切面可见的褐色内生菌丝
Fig. 1
Morphological characteristics of Mattirolomyces terfezioides.
A-C: Fresh ascomata and gleba; D: Peridium structure and puberulum on the surface; E, F: Ascus and globular ascospore with spiny ornamentation; G: Fresh ascomata enclosed in the soil block of host plant root; H: The young root tip expanded into a rod like ectomycorrhizal structure; I: Brown endophytic hyphae in cross sections of host plant root of Prunus persica.
生境及分布:中国,河北省保定市满城县杨园村,人工桃林。
凭证标本号:河北省保定市满城县杨园,2020年8月20日,陈娟475;2020年9月18日,陈娟476;2020年10月31日,陈娟477、478。
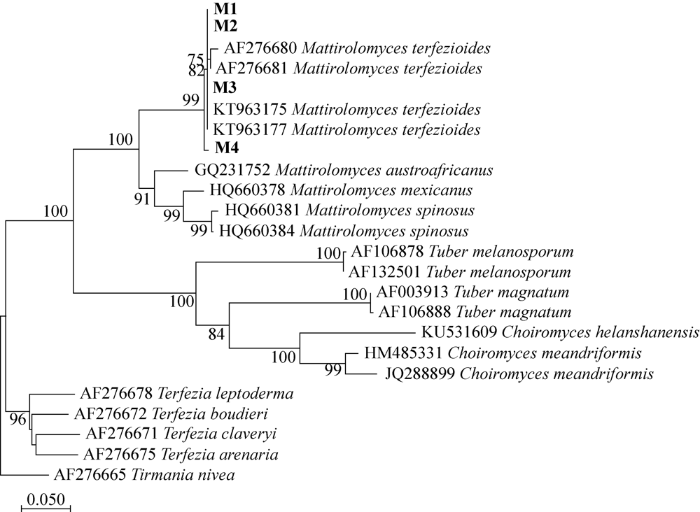
2.2 分子系统学分析
基于ITS序列构建的系统发育树(图2)表明在河北新发现的块状真菌与此前报道的M. terfezioides聚在同一支,且有99%的支持率。本研究结果为该种在我国的分布补充了新的标本证据。
图2
图2
基于ITS序列采用邻接法(neighbor-joining method)构建的系统发育树分支上的数字>50%的靴带支持率
黑体表示本研究新获得的序列
Fig. 2
The phylogenetic tree constructed with neighbor-joining method based on ITS sequence.
The boldface indicates sequences acquired in this study.
2.3 挥发性化合物
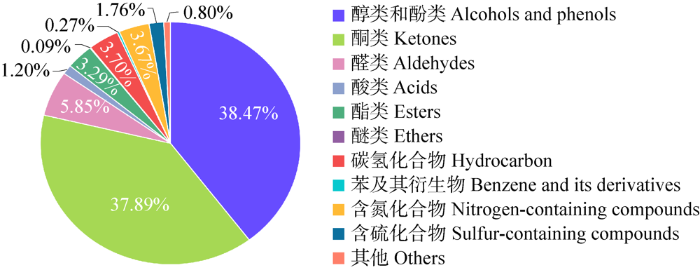
本研究从地菇状马蒂菌子囊果中共检测出68种化合物,其组成及相对含量见表1,主要包括醇类及酚类化合物9种(38.47%)、酮类8种(37.89%)、醛类13种(5.85%)、酯类7种(3.29%)、酸类6种(1.20%)、醚类1种(0.09%)、碳氢化合物10种(3.70%)、苯及其衍生物2种(0.27%)、含氮化合物6种(3.67%)和含硫化合物3种(1.76%) (图3)。其中醛类、酮类、酸类、酯类、醚类和醇类及酚类化合物等含氧化合物占比很高(86.79%)。从相对含量来看,含量最高的物质为3-辛酮(37.19%),其次为1-辛烯-3-醇和3-辛醇,含量分别为22.67%和12.53%,以上3种成分含量远远高于其他挥发性成分含量,其他化合物含量均低于3%。含量介于1%-3%的挥发性成分有6种,分别为:2-氨基-5-甲基苯甲酸(2.28%)、α-亚乙基-苯乙醛(2.21%)、十三烷(2.18%)、苯乙醇(1.37%)、顺-9-十六碳烯酸乙酯(1.32%)、3-甲硫基丙醛(1.06%)。从地菇状马蒂菌子囊果中也检测到3种含硫化合物,分别为3-甲硫基丙醛、2-异丁基-5-丙基-噻吩(0.55%)、甲基乙基硫醚(0.15%)。
表1 地菇状马蒂菌子囊果挥发性化合物GC-MS分析 (n=3)
Table 1
| 序号 No. | 化合物 Compound | 分子式 Molecular formula | 分子量 Molecular weight | CAS | 相对含量 Relative content (%) |
|---|---|---|---|---|---|
| 醇类及酚类化合物Alcohols and phenols | |||||
| 1 | 1-辛烯-3-醇 1-Octen-3-ol | C8H16O | 128.21 | 3391-86-4 | 22.67±17.61 |
| 2 | 3-辛醇 3-Octanol | C8H18O | 130.23 | 20296-29-1 | 12.53±11.28 |
| 3 | 苯乙醇 Phenylethyl alcohol | C8H10O | 122.16 | 60-12-8 | 1.37±0.73 |
| 4 | 2,4,7,9-四甲基-5-癸炔-4,7-二醇 2,4,7,9-Tetramethyl-5-decyn-4,7-diol | C14H26O2 | 226.35 | 126-86-3 | 0.51±0.88 |
| 5 | 3-戊炔-1-醇 3-Pentyn-1-ol | C5H8O | 84.12 | 10229-10-4 | 0.50±0.87 |
| 6 | 反式-2-辛烯-1-醇 2-Octen-1-ol, (E)- | C8H16O | 128.21 | 18409-17-1 | 0.37±0.64 |
| 7 | 2-甲基丁醇 1-Butanol, 2-methyl- | C5H12O | 88.15 | 137-32-6 | 0.27±0.24 |
| 8 | 2-乙基己醇 1-Hexanol, 2-ethyl- | C8H18O | 130.23 | 104-76-7 | 0.16±0.28 |
| 9 | 橙花醇 2,6-Octadien-1-ol, 3,7-dimethyl-, (Z)- | C10H18O | 154.25 | 106-25-2 | 0.09±0.15 |
| 酮类化合物Ketones | |||||
| 10 | 3-辛酮 3-Octanone | C8H16O | 128.21 | 106-68-3 | 37.19±6.96 |
| 11 | 2-环戊烯酮 2-Cyclopenten-1-one | C5H6O | 82.1 | 930-30-3 | 0.25±0.43 |
| 12 | 香叶基丙酮 5,9-Undecadien-2-one, 6,10-dimethyl-, (E)- | C13H22O | 194.31 | 3796-70-1 | 0.13±0.04 |
| 13 | 甲基壬基甲酮 2-Undecanone | C11H22O | 170.29 | 112-12-9 | 0.10±0.09 |
| 14 | 2-(1-甲基丙基)环己酮 2-Sec-butylcyclohexanone | C10H18O | 154.25 | 14765-30-1 | 0.09±0.16 |
| 15 | 2,5-二羟基-3-甲氧基-6-甲基-2,5-环己二烯-1,4-二酮 2,5-Cyclohexadiene-1,4-dione, 2,5-dihydroxy-3-methoxy-6-methyl | C8H8O5 | 184.15 | 85-23-4 | 0.06±0.10 |
| 16 | 4,5-辛二酮 4,5-Octanedione | C8H14O2 | 142.2 | 5455-24-3 | 0.04±0.07 |
| 17 | 3-乙基-4-庚酮 4-Heptanone, 3-ethyl- | C9H18O | 142.24 | 1528-25-2 | 0.03±0.05 |
| 醛类化合物Aldehydes | |||||
| 18 | α-亚乙基-苯乙醛 Benzeneacetaldehyde, alpha-ethylidene- | C10H10O | 146.19 | 4411-89-6 | 2.21±1.37 |
| 19 | 苯乙醛 Benzeneacetaldehyde | C8H8O | 120.15 | 122-78-1 | 0.86±0.57 |
| 20 | 苯甲醛 Benzaldehyde | C7H6O | 106.12 | 100-52-7 | 0.70±0.27 |
| 21 | 反-2-辛烯醛 2-Octenal, (E)- | C8H14O | 126.2 | 2548-87-0 | 0.64±0.37 |
| 22 | 糠醛 Furfural | C5H4O2 | 96.08 | 98-01-1 | 0.29±0.51 |
| 23 | (Z)-3,7-二甲基-2,6-辛二烯醛 2,6-Octadienal, 3,7-dimethyl-, (Z)- | C10H16O | 152.23 | 106-26-3 | 0.28±0.48 |
| 24 | 壬醛 Nonanal | C9H18O | 142.24 | 124-19-6 | 0.26±0.44 |
| 25 | 异戊醛 Butanal, 3-methyl- | C5H10O | 86.13 | 590-86-3 | 0.21±0.19 |
| 26 | 可卡醛 5-Methyl-2-phenyl-2-hexenal | C13H16O | 188.27 | 21834-92-4 | 0.16±0.28 |
| 27 | 2-乙基苯甲醛 Benzaldehyde, 2-ethyl- | C9H10O | 134.18 | 22927-13-5 | 0.10±0.18 |
| 28 | 反式-2,4-癸二烯醛 2,4-Decadienal | C10H16O | 152.23 | 2363-88-4 | 0.06±0.10 |
| 29 | 间苯二甲醛 Isophthalaldehyde | C8H6O2 | 134.13 | 626-19-7 | 0.05±0.09 |
| 30 | (Z,Z)-10,12-十六二烯醛 (Z,Z)-10,12-Hexadecadienal | C16H28O | 236.39 | 96348-46-8 | 0.03±0.05 |
| 酸类化合物Acids | |||||
| 31 | 醋酸 Acetic acid | C2H4O2 | 60.05 | 64-19-7 | 0.77±0.69 |
| 32 | 辛酸 Octanoic acid | C8H16O2 | 144.21 | 124-07-2 | 0.19±0.33 |
| 33 | 4-甲氧基-3,5-二羟基苯甲酸 4-Methoxy-3,5-dihydroxybenzoic acid | C8H8O5 | 184.15 | 4319-02-2 | 0.07±0.13 |
| 34 | 亚油酸 (Z,Z)-9,12-octadecadienoic acid | C18H32O2 | 280.45 | 60-33-3 | 0.06±0.11 |
| 35 | 棕榈油酸 Palmitoleic acid | C16H30O2 | 254.41 | 373-49-9 | 0.06±0.10 |
| 36 | 苯甲酸 Benzoic acid | C7H6O2 | 122.12 | 65-85-0 | 0.05±0.09 |
| 37 | 顺-9-十六碳烯酸乙酯 Ethyl 9-hexadecenoate | C18H34O2 | 282.46 | 56219-10-4 | 1.32±1.48 |
| 38 | 棕榈酸乙酯 Hexadecanoic acid, ethyl ester | C18H36O2 | 284.48 | 628-97-7 | 0.72±0.71 |
| 39 | (Z)-3,7-二甲基-2,6-辛二烯-1-醇甲酸酯 2,6-Octadien-1-ol, 3,7-dimethyl-, formate, (Z)- | C11H18O2 | 303.35 | 2142-94-1 | 0.59±1.03 |
| 40 | 庚酸,2-丙基甲酯 Heptanoic acid, 2-propyl- methyl ester | C11H22O2 | 186.29 | 56247-53-1 | 0.49±0.85 |
| 41 | 油酸乙酯 Ethyl oleate | C20H38O2 | 310.51 | 111-62-6 | 0.09±0.16 |
| 42 | 顺式-4-羟基-6-十二烯酸内酯 2(3H)-Furanone, dihydro-5-(2-octenyl)-, (Z)- | C12H20O2 | 196.29 | 18679-18-0 | 0.05±0.09 |
| 43 | 丁酸丁酯 Butanoic acid, butyl ester | C8H16O2 | 144.21 | 109-21-7 | 0.03±0.05 |
| 醚类化合物Ethers | |||||
| 44 | 乙基烯丙基醚 Allyl ethyl ether | C5H10O | 86.13 | 557-31-3 | 0.09±0.16 |
| 碳氢化合物Hydrocarbon | |||||
| 45 | 十三烷 Tridecane | C13H28 | 184.36 | 629-50-5 | 2.18±3.78 |
| 46 | 6,6-二甲基庚-2,4-二烯 6,6-Dimethylhepta-2,4-diene | C9H16 | 124.22 | 263008-30-6 | 0.88±0.23 |
| 47 | 8-十七烷烯 8-Heptadecene | C17H34 | 238.45 | 16369-12-3 | 0.20±0.35 |
| 48 | 十四烷 Tetradecane | C14H30 | 198.39 | 629-59-4 | 0.11±0.19 |
| 49 | 2,3,3-三甲基-1,4-戊二烯 2,3,3-Trimethyl-1,4-pentadiene | C8H14 | 110.2 | 756-02-5 | 0.07±0.13 |
| 50 | 3,5,5-三甲基-2-己烯 2-Hexene, 3,5,5-trimethyl- | C9H18 | 126.24 | 26456-76-8 | 0.06±0.11 |
| 51 | 1-(1-甲基乙基)-2-壬基-环丙烷 Cyclopropane, 1-(1-methylethyl)-2-nonyl- | C15H30 | 210.4 | 41977-39-3 | 0.06±0.10 |
| 52 | 十二烷 Dodecane | C12H26 | 170.33 | 112-40-3 | 0.05±0.09 |
| 53 | 9-甲基双环[3.3.1]壬烷 9-Methylbicyclo[3.3.1]nonane | C10H18 | 138.25 | 25107-01-1 | 0.05±0.09 |
| 54 | 正十七烷 Heptadecane | C17H36 | 240.47 | 629-78-7 | 0.04±0.06 |
| 苯及其衍生物Benzene and its derivatives | |||||
| 55 | 3,4,5-三甲氧基甲苯 3,4,5-Trimethoxytoluene | C10H14O3 | 182.22 | 6443-69-2 | 0.14±0.12 |
| 56 | 3-苯基戊烷 Benzene, (1-ethylpropyl)- | C11H16 | 148.24 | 1196-58-3 | 0.13±0.23 |
| 含氮化合物Nitrogen-containing compounds | |||||
| 57 | 2-氨基-5-甲基苯甲酸 2-Amino-5-methylbenzoic acid | C8H9NO2 | 151.16 | 2941-78-8 | 2.28±3.36 |
| 58 | 2,4-二氨基-6-丁基氨基-1,3,5-三嗪 N-Butylmelamine | C7H14N6 | 182.23 | 5606-24-6 | 0.88±1.53 |
| 59 | 2,4-二甲基咪唑 1H-Imidazole, 2,4-dimethyl- | C5H8N2 | 96.13 | 930-62-1 | 0.29±0.51 |
| 60 | 吲哚 Indole | C8H7N | 117.15 | 120-72-9 | 0.10±0.17 |
| 61 | 川芎嗪 Pyrazine, tetramethyl- | C8H12N2 | 136.19 | 1124-11-4 | 0.07±0.12 |
| 62 | N,N-二丁基甲酰胺 Formamide, N, N-dibutyl- | C9H19NO | 157.25 | 761-65-9 | 0.05±0.09 |
| 含硫化合物Sulfur-containing compounds | |||||
| 63 | 3-甲硫基丙醛 Methional | C4H8OS | 104.17 | 3268-49-3 | 1.06±0.94 |
| 64 | 2-异丁基-5-丙基-噻吩 Thiophene, 2-isobutyl-5-propyl- | C11H18S | 182.33 | 4861-63-6 | 0.55±0.95 |
| 65 | 甲基乙基硫醚 Ethane, (methylthio)- | C3H8S | 76.16 | 624-89-5 | 0.15±0.05 |
| 其他化合物Others | |||||
| 66 | 2,6-二氟苯酚 2,6-Difluorophenol | C6H4F2O | 130.09 | 28177-48-2 | 0.64±1.10 |
| 67 | 1-溴-2-甲基癸烷 Decane, 1-bromo-2-methyl- | C11H23Br | 235.2 | 127839-47-8 | 0.10±0.18 |
| 68 | 氢氯化蒎烯 Bicyclo[2.2.1]heptane, 2-chloro-1,7,7-trimethyl-, (1R-endo)- | C10H17Cl | 172.7 | 30462-53-4 | 0.06±0.10 |
图3
图3
地菇状马蒂菌子囊果中挥发性成分的种类及其含量
Fig. 3
The variety and content of the volatile compounds in Mattirolomyces terfezioides ascocarps.
3 讨论
我国在20世纪80年代以刘波先生为代表集中开展了地下真菌资源调查研究,尤其在腹菌类真菌方面的研究成果显著(刘波和陶恺 1989)。90年代随着块菌贸易在我国西南地区兴起,地下真菌的研究重点转移到真块菌类群即块菌属Tuber的分类和系统学。20多年来,块菌属的一系列新种在我国相继报道,显示了我国丰富的地下真菌资源多样性(Chen et al. 2011;Fan et al. 2013;Wan et al. 2017a,2017b;Wang et al. 2016,2017)。然而,对其他地下真菌资源新采集记录很少有补充和关注。近几年,随着对地下真菌的广泛调查,先后有猪块菌Choiromyces helanshanensis Juan Chen & P.G. Liu (Chen et al. 2016)、地菇状马蒂菌M. terfezioides (Wang et al. 2017)以及史蒂芬块菌属 Stephensia Tul. & C. Tul. (Wang et al. 2016)等稀有块菌资源在我国发现和报道,进一步确认了这些类群在中国的分布,同时为早期研究补充了新的标本及数据资料。
地菇属Terfezia曾报道在北美分布,然而Kovács et al. (2011b)的分子系统学结果表明原鉴定为T. spinosa 的种被归到M. terfezioides,而T. longii Gilkey则升级为一个新属的模式种即Stouffera longii (Gilkey) Kovács & Trappe,因此排除了地菇属真菌在北美的分布。刘波等(2002)在《中国大型地下真菌》一文中提到网孢地菇Terfezia terfezioides在我国的分布,记录其主要分布于北京、河北、山西和河南等地。然而,大量文献表明,地菇菌科真菌主要分布于干旱和半干旱的沙漠地区,通常与半日花科Cistaceae植物共生;因此,以往文献中记载的地菇属真菌极有可能不是“真正”的地菇属真菌,Wang et al. (2017)对早期存放在中国科学院微生物研究所标本馆(HMAS)馆藏鉴定为T. terfezioides的标本的分子序列表明,其应该归属于马蒂菌属Mattirolomyces,因此就沙漠块菌地菇属Teferzia 是否在我国有分布尚存疑待证实。
大多数已有研究推测马蒂菌属Mattirolomyces是外生菌根真菌(Healy 2003),有关地菇状马蒂菌M. terfezioides的宿主植物,最早基于馆藏标本的生境记录以及群落学推断其可能与刺槐R. pseudoacacia形成菌根共生关系(Király et al. 1992)。随后,Bratek et al. (1996)采用M. terfezioides接种R. pseudoacacia,并在显微镜下观察其菌根,未见菌套和哈氏网,菌根的形态特征是以有隔菌丝定殖在根皮层细胞。随后,Kovács et al. (2007)在鉴定M. terfezioides的宿主植物时,也在R. pseudoacacia根部发现类似结构。本研究采集M. terfezioides时发现其在人工种植的桃树林地,采集根样镜检发现桃树须根根端有膨大结构,外形似外生菌根,但徒手切片观察的结果在根被细胞外没有发现大量的菌丝聚集,未观察到典型的外生菌根结构(即菌套和哈氏网),仅在根内发现菌丝。下一步将桃树幼苗接种M. terfezioides进一步明确其宿主植物及菌根状况。
新鲜的地菇状马蒂菌有很浓郁的甜香味,尝起来非常甜,当地居民将其误认为“白块菌”而采食,但产量不大,子囊果零星分布于桃树下。本研究检测了其新鲜样本的挥发性成分,并分析其与新鲜块菌属Tuber成员挥发性成分的共性和差异性。本研究发现其子囊果挥发性化合物的组成与块菌属成员相似,但在含量上具有显著的差异。研究表明,C8挥发性化合物(如1-辛烯-3-醇、3-辛酮、3-辛醇、2-辛烯-1-醇和反式-2-辛烯醛)是块菌属真菌的主要芳香物质,它们是由C16和C22等不饱和脂肪酸通过酰基水解酶、脂肪氧合酶和裂解酶类等酶及酶系的催化形成(杨生兵等 2013;Lu et al. 2021)。地菇状马蒂菌子囊果同样含有丰富的C8挥发性化合物,占总挥发性成分含量的75%左右,显著高于块菌属真菌中C8挥发性成分含量(Strojnik et al. 2020)。同时,地菇状马蒂菌子囊果中3-辛酮的含量高于1-辛烯-3-醇,这与块菌属真菌挥发性成分特征不一致,在块菌属真菌中1-辛烯-3-醇含量通常远高于3-辛酮(Lu et al. 2021)。此外,块菌属真菌中含有丰富的含氧化合物,如甲酸丁酯、对苯二甲醚和丁酮等,但这些成分均未在地菇状马蒂菌子囊果中检测到。芳香化合物也是真菌气味芳香的关键原因,如2-苯乙醇具有特征的玫瑰气味,苯甲醛散发着苦杏仁味道,以上两种物质均在地菇状马蒂菌子囊果中检测到(1.37%、0.7%) (Vahdatzadeh et al. 2015)。间甲基苯甲醚普遍存在于块菌属真菌中,甚至T. mesentericum、凹陷块菌T. excavatum、冬块菌T. brumale和印度块菌T. indicum等多种块菌中均具有较高含量的间甲基苯甲醚(28%-70%) (Strojnik et al. 2020),但并未在地菇状马蒂菌子囊果中检测到此化合物。含硫挥发物是块菌属真菌最重要的香气物质,是人们能识别块菌香气的重要原因。块菌属真菌子囊果中最常见的含硫挥发性物质是二甲基硫醚,迄今为止,在已调查块菌属物种中,85%的物种中都能检测到二甲基硫醚,但在地菇状马蒂菌子囊果中并未检测到;其他仅存在于一两种块菌中的含硫化合物,如2-甲基-4,5-二氢噻吩、3-甲基-4,5-二氢噻吩、2,4-二噻戊烷和2-甲基呋喃-3-硫醇也并未在地菇状马蒂菌子囊果中检出(Vahdatzadeh et al. 2015)。作为黑块菌的芳香气味成分之一,3-甲硫基丙醛存在于地菇状马蒂菌子囊果中,该化合物具有马铃薯气味,且含量很低(Culleré et al. 2010),推测其并不能对地菇状马蒂菌的香气起到关键作用。地菇状马蒂菌子囊果中含硫化合物含量较低(<2%),普遍低于所有块菌属真菌中含硫化合物含量,这一结果有助于解释地菇状马蒂菌和块菌属真菌气味的差异。
地菇状马蒂菌与块菌属中的白块菌类在子囊果宏观形态特征上高度相似,但是子囊孢子的形状和特征与块菌属成员显著不同,且其成熟期短,直至腐烂掉,产孢组织白色至黄白色,不变黑色,这与块菌属成员显著不同。挥发性化合物的组成也与块菌属成员有显著差异,但是个别成分上有交叠,也一定程度上解释了其具有类似块菌香气的原因,可能反应在系统亲缘关系上与块菌属成员比较接近。这是对该类地下真菌资源的化学组成分析的首次记录,将为人们更好地认识块菌,保护和利用我国地下真菌资源奠定基础。
在欧洲,M. terfezioides 被认为是可食用真菌并探索尝试进行人工栽培,但是它的市场价值目前被认为低于地中海地区的其他商业块菌如沙漠块菌和块菌属真菌。在我国,据采集地人介绍,M. terfezioides在当地是可食用的,生吃并未引起不适或中毒。然而,由于其稀有少见,且产量不大,有可食用记录相对较少,因此,关于其营养成分的分析目前正在进行。
全球估计有4 500-5 500种地下真菌,目前已经描述种类大概有700余种,随着地下真菌多样性被持续发现,新的数据资料将拓宽我们对这类真菌的起源、演化和地理分布等方面的认识,更好地保护、开发和利用。
致谢
感谢河北省保定市满城杨园严迎春先生提供样品资料及野外考察便利。
参考文献
Mycorrhizae between black locust (Robinia pseudoacacia) and Terfezia terfezioides
DOI:10.1007/s005720050136 URL [本文引用: 1]
Species recognition and cryptic species in the Tuber indicum complex
DOI:10.1371/journal.pone.0014625 URL [本文引用: 1]
Choiromyces helanshanensis sp. nov., a new species of subterranean truffle from China
DOI:10.1016/j.myc.2016.04.001 URL [本文引用: 1]
Characterisation of aroma active compounds in black truffles (Tuber melanosporum) and summer truffles (Tuber aestivum) by gas chromatography-olfactometry
DOI:10.1016/j.foodchem.2010.02.024 URL [本文引用: 1]
A revised checklist of edible fungi in China
Tuber in China: T. sinopuberulum and T. vesicoperidium spp. nov
DOI:10.5248/121.255 URL [本文引用: 1]
ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts
We have designed two taxon-selective primers for the internal transcribed spacer (ITS) region in the nuclear ribosomal repeat unit. These primers, ITS1-F and ITS4-B, were intended to be specific to fungi and basidiomycetes, respectively. We have tested the specificity of these primers against 13 species of ascomycetes, 14 of basidiomycetes, and 15 of plants. Our results showed that ITS4-B, when paired with either a 'universal' primer ITS1 or the fungal-specific primer ITS1-F, efficiently amplified DNA from all basidiomycetes and discriminated against ascomycete DNAs. The results with plants were not as clearcut. The ITS1-F/ITS4-B primer pair produced a small amount of PCR product for certain plant species, but the quantity was in most cases less than that produced by the 'universal' ITS primers. However, under conditions where both plant and fungal DNAs were present, the fungal DNA was amplified to the apparent exclusion of plant DNA. ITS1-F/ITS4-B preferential amplification was shown to be particularly useful for detection and analysis of the basidiomycete component in ectomycorrhizae and in rust-infected tissues. These primers can be used to study the structure of ectomycorrhizal communities or the distribution of rusts on alternate hosts.
Mattirolomyces tiffanyae, a new truffle from Iowa, with ultrastructural evidence for its classification in the Pezizaceae
A new species of hypogeous Pezizales, Mattirolomyces tiffanyae, is described and illustrated. Its asci are typically three-spored, an unusually small number in the non-Tuber Pezizales. Ascus septal pore ultrastructure consists of a uni- or bi-convex band, which suggests an affinity with the Pezizaceae. Secondary spore-wall development is similar to that of Peziza, and several species of hypogeous Pezizaceae.
The arenicolous truffle (Terfezia terfezioides)
The diversity of Terfezia desert truffles: new species and a highly variable species complex with intrasporocarpic nrDNA ITS heterogeneity
DOI:10.3852/10-312 URL [本文引用: 1]
Identification of host plants and description of sclerotia of the truffle Mattirolomyces terfezioides
DOI:10.1007/s11557-006-0520-y URL [本文引用: 3]
Terfezia disappears from the American truffle mycota as two new genera and Mattirolomyces species emerge
DOI:10.3852/10-273 URL [本文引用: 2]
Distribution of Tuber indicum in northeastern China and its ecological significance
Aroma profile of two commercial truffle species from Yunnan and Sichuan, China: inter- and intraspecific variability and shared key compounds
DOI:10.1016/j.fshw.2021.02.005 URL [本文引用: 2]
A brief history of underground fungi research and the known species in China
Molecular phylogenetic analysis of Peziza and related genera
DOI:10.1080/00275514.1999.12061087 URL [本文引用: 1]
Molecular phylogeny of truffles (Pezizales: Terfeziaceae, Tuberaceae) derived from nuclear rDNA sequence analysis
Extensive morphological convergence or divergence, a common occurrence in fungi, tends to obscure recognition of phylogenetic relationships among Pezizales, widespread filamentous Ascomycetes with either enclosed underground (hypogeous) or exposed (epigeous) fruit bodies, that often establish mutualistic interactions with arboreous plants. Focusing on hypogeous Pezizales commonly known as truffles, we sequenced the 18S rDNA from nine species belonging to three different families (Tuberaceae, Terfeziaceae, and Balsamiaceae). A data set consisting of 1700 secondary structure-aligned sites, including 24 homologous sequences from the GenBank DNA database and using three reconstruction methods, was employed to infer phylogenies in an interval ranging from the subordinal to the subgeneric level. As revealed by the 18S phylogenetic scheme, Balsamiaceae represent a monophyletic clade, comprising the hypogeous taxa Balsamia and Barssia, nested within Helvellaceae. Similarly, the terfeziacean genera Pachyphloeus and Terfezia constitute together with Cazia a distinct hypogeous clade nested within Pezizaceae. The lack of clustering between Terfezia arenaria and Terfezia terfezioides strongly supports the reassignment of the latter taxon to the original monotypic genus Mattirolomyces. Within Tuberaceae, which are sister to the highly evolved Helvellaceae, the genus Tuber cannot be considered monophyletic if Choiromyces is recognized. The paraphyly of Tuber and other relationships that were not supported by high bootstrap values, nor corroborated by morphological evidence, were supported by a parallel analysis of the faster evolving internal transcribed spacer (ITS) rDNA. Distinct episodes of fruit body morphology shifts are discernable in the 18S rDNA phylogenetic tree. In all cases, the shift from an epigeous to a hypogeous form is the most parsimonious interpretation of character transformation, without any instance of character reversal.Copyright 1999 Academic Press.
Species and geographic variability in truffle aromas
DOI:10.1016/j.fct.2020.111434
PMID:32442473
[本文引用: 2]

The gastronomic relevance and price of truffles are related mainly to its unique aroma. In this study, we explore the impact that different volatile compounds have on the aroma quality of fresh truffles using gas chromatography-mass spectrometry (GC-MS). Four hundred sixty fresh ascocarps of nine truffle species (Tuber aestivum, Tuber magnatum, Tuber melanosporum, Tuber mesentericum, Tuber brumale, Tuber excavatum, Tuber rufum, Tuber indicum and Tuber macrosporum) harvested in 2018/19 and 2019/2020 from 11 different countries (Slovenia, Croatia, Bosnia in Herzegovina, Macedonia, Italy, Spain, France, United Kingdom, Germany, Poland and China) were collected. Our investigation included the classification of species based on their aroma profile, a study of the differences in the volatile organic composition of truffle species over a geographical area, and, in more detail, a study of T. aestivum from four natural truffle growing sites in Slovenia. Our models can distinguish between groups, with small classification error. These models could form the basis of a predictive framework to detect fraud concerning truffle products and to determine the influence of different growing parameters on the aroma profile of truffles.Copyright © 2020 The Authors. Published by Elsevier Ltd.. All rights reserved.
A synopsis of the Carbomycetaceae and Terfeziaceae (Tuberales)
DOI:10.1016/S0007-1536(71)80083-9 URL [本文引用: 1]
Comparative taxonomy of desert truffles of the Australian outback and the African Kalahari
DOI:10.1007/s11557-009-0612-6 URL [本文引用: 1]
Validation of the new combination Mattirolomyces austroafricanus
DOI:10.1007/s11557-009-0631-3 URL [本文引用: 1]
The role of the microbiome of truffles in aroma formation: a meta-analysis approach
DOI:10.1128/AEM.01098-15
PMID:26187969
[本文引用: 2]

Truffles (Tuber spp.) are ascomycete subterraneous fungi that form ectomycorrhizas in a symbiotic relationship with plant roots. Their fruiting bodies are appreciated for their distinctive aroma, which might be partially derived from microbes. Indeed, truffle fruiting bodies are colonized by a diverse microbial community made up of bacteria, yeasts, guest filamentous fungi, and viruses. The aim of this minireview is two-fold. First, the current knowledge on the microbial community composition of truffles has been synthesized to highlight similarities and differences among four truffle (Tuber) species (T. magnatum, T. melanosporum, T. aestivum, and T. borchii) at various stages of their life cycle. Second, the potential role of the microbiome in truffle aroma formation has been addressed for the same four species. Our results suggest that on one hand, odorants, which are common to many truffle species, might be of mixed truffle and microbial origin, while on the other hand, less common odorants might be derived from microbes only. They also highlight that bacteria, the dominant group in the microbiome of the truffle, might also be the most important contributors to truffle aroma not only in T. borchii, as already demonstrated, but also in T. magnatum, T. aestivum, and T. melanosporum. Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Two novel Tuber species (Tuberaceae, Pezizales) in the Latisporum Group from China
DOI:10.1016/j.myc.2017.02.006 URL [本文引用: 1]
Three excavatum species Tuber badium, T. depressum and T. verrucosivolvum from Sichuan Province, China
DOI:10.11646/phytotaxa.296.3.2 URL [本文引用: 1]
Molecular and morphological data confirmed the presence of the rare species Mattirolomyces terfezioides in China
DOI:10.1016/j.pld.2016.10.002 URL [本文引用: 4]
Stephensia (Pyronemataceae, Pezizales), a new record of Chinese hypogeous Ascomyctete
Resource diversity of Chinese macrofungi: edible, medicinal and poisonous species
DOI:10.1007/s13225-019-00432-7 URL [本文引用: 1]
Analysis of volatile compounds in fruiting bodies and submergedly cultured mycelia of Grifola frondosa