灵芝生活史有担孢子、菌丝体、原基和子实体等4个阶段,一般在子实体成熟后菌盖会弹射出淡雾状卵形的生殖细胞(即灵芝孢子),因其外观呈粉末状而称之为灵芝孢子粉(Ma et al. 2012;孙超等 2013;王金艳等 2016)。灵芝Ganoderma lingzhi是一种药用价值很高的大型担子菌,在中国的应用历史悠久(Cao et al. 2012;戴玉成等 2013;王金艳等 2016)。研究表明灵芝富含多种次生代谢产物,其中部分化合物具有一定的药用功能,如灵芝三萜类化合物可抗肿瘤(闫征等 2017)、酚类和多糖类化合物具有抗氧化(Heleno et al. 2012)、多糖类化合物还具有免疫调节作用(Wang et al. 2012)。随着药理活性得到广泛认可,灵芝已先后被《中华人民共和国药典(2000年版)》(国家药典委员会 2000)和《美国草药药典与治疗概要(American Herbal Pharmacopoeia and Therapeutic Compendium)》(Sanodiya et al. 2009)收载。
灵芝孢子具有灵芝全部的遗传物质(周亚杰等 2018),是灵芝的精华所在。灵芝孢子不仅具有很好的调节神经(Zhou et al. 2012)、免疫(Wang et al. 2012)、消化(胡宗苗等 2016)、呼吸(廖逸茹等 2019)及心血管系统(王超等 2016)等作用,其药用功效甚至是灵芝子实体的75倍(柴玉 2018)。
目前对灵芝孢子粉次生代谢产物的研究主要以大分子的活性物质如灵芝多糖、三萜类物质为主,在分离萃取方法上多采用提取程度较低的水或醇等单一溶剂萃取(王金艳等 2016;宋玮等 2019),限制了灵芝孢子粉中活性成分的充分分离。在测定方法上,以测定大分子活性物质为主的液相色谱法(王金艳等 2016)运用较多,而较适合小分子物质测定的气相色谱法运用则相对较少。为了系统、全面地了解灵芝孢子粉中的化学成分,本研究根据相似相溶原理,选取石油醚、乙酸乙酯、丙酮、乙醇、甲醇、水等6种不同极性溶剂对破壁灵芝孢子粉进行索氏分级萃取,并同时采用气相色谱-质谱联用(gas chromatography-mass spectrometry,GC/MS)和超高效液相色谱-四级杆高分辨飞行时间质谱(ultra-performance liquid chromatography coupled with electrospray time-of-flight/mass spectrometry,UPLC-Q-TOF/MS)两种测定技术,分析和鉴定灵芝孢子粉中次生代谢产物的成分和结构,为灵芝孢子粉化合物谱图库的完善以及灵芝的药理作用和模式真菌等相关研究提供参考。
1 材料与方法
1.1 材料、试剂和主要仪器
灵芝孢子粉(购自安徽省金寨县活力源菌业有限公司)采用超微粉碎机破壁处理。石油醚(西陇化工股份有限公司)为分析纯;甲醇(上海星可高纯溶剂有限公司)为色谱纯;Agilent 7890A-5975C型气相色谱-质谱联用仪(美国Agilent公司);UPLCH-CLASS/QTOF G2-XS型超高效液相色谱串联四极杆飞行时间质谱仪(美国Waters公司);1810D型超纯水净化系统(申生科技有限公司);TG16-WS型台式高速离心机(上海卢湘仪离心机仪器有限公司);DHG-9101-2A型电热恒温鼓风干燥箱(上海三发科学仪器有限公司);AUY220型电子天平(岛津科技有限公司);XL-600B型多功能粉碎机(永康市小宝电器有限公司)。
1.2 供试样品溶液的制备
1.2.1 索氏提取:样品处理及萃取参照甘卓亭等(2019)的方法。灵芝孢子粉经石油醚、乙酸乙酯、丙酮、乙醇、甲醇和水等6级萃取剂逐级提取,提取物分别标记为PE、EA、AE、EL、ML和DI,离心处理后备用。
1.3 检测方法
1.3.1 GC/MS检测:GC/MS参数设置参照甘卓亭等(2019)的方法。采用MSD ChemStation软件处理数据,通过比对所得质谱图与NIST08标准谱图鉴定化合物,采用归一化法对所检测的化合物进行定量分析。
1.3.2 UPLC-Q-TOF/MS检测:取待测提取物1μL,流速0.3mL/min,色谱柱ACQUITY HSS T3(2.1mm× 100mm,1.8μm);柱温30℃;流动相为0.1%的甲酸水溶液(A)-乙腈(B);梯度洗脱条件:0-5min,95% A;5-50min,95% A;50-55min,5% A;55-57min,5% A;57-60min,95% A。ESI电喷雾离子源,正离子模式检测。离子源温度120℃;脱溶剂气温度80℃;脱溶剂气流量900L/h;碰撞能量2-30V;毛细管电压3kV;进样锥电压40V;锥气孔流量50L/h;一级质谱母离子扫描范围:m/z 50-1 200;二级质谱碎片离子扫描范围:m/z 50-1 000。利用Masslynx软件中的Massfragment软件并结合相关文献分析和鉴定化合物的分子量与分子结构式。
2 结果与分析
2.1 灵芝孢子粉萃取物的GC/MS分析
Table 1 Compounds of extracts from Ganoderma lingzhi spore powder using petroleum ether, ethyl acetate, acetone, ethanol and methanol as solvents analysed by GC-MS
| 分类 Type | 化合物 Compound | 结构 Structural formula | 化学式 Molecular formula | 匹配度 Matching degree (%) | 相对含量 Relative content (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| PE | EA | AE | EL | ML | |||||
| 酸类 Acids | |||||||||
| A1 | 壬酸 Nonanoic acid | C9H18O2 | 95 | - | 0.51 | 0.17 | - | - | |
| A2 | 正十五酸 Pentadecanoic acid | C15H30O2 | 99 | 0.31 | 0.41 | 0.45 | - | 0.36 | |
| A3 | (E)-9-十八碳烯酸 (E)-octadec-9-enoicacid | C18H34O2 | 99 | - | - | 33.30 | - | - | |
| A4 | (Z)-6-十八碳烯酸 (Z)-octadec-6-enoic acid | C18H34O2 | 99 | - | - | - | - | 26.24 | |
| A5 | (E)-6-十八碳烯酸 (E)-octadec-6-enoic acid | C18H34O2 | 99 | - | 36.47 | - | 40.01 | - | |
| A6 | (Z)-9-十六碳烯酸 (Z)-hexadec-9-enoic acid | C16H30O2 | 99 | - | 1.16 | - | - | - | |
| A7 | 油酸 Oleic acid | C18H34O2 | 99 | 25.61 | - | - | - | - | |
| A8 | 亚油酸 (9Z,12Z)-octadeca-9,12-dienoic acid | C18H32O2 | 97 | - | - | - | - | 17.34 | |
| A9 | 硬脂酸 Stearic acid | C18H36O2 | 99 | 1.85 | 2.33 | 2.33 | - | 2.13 | |
| A10 | 棕榈酸 Palmitic acid | C16H32O2 | 99 | 4.10 | 9.61 | - | 13.49 | 12.52 | |
| 合计 Subtotal | 31.87 | 50.49 | 36.87 | 53.50 | 58.58 | ||||
| 酯类 Esters | |||||||||
| B1 | (Z)-9-十六烯酸甲酯 Methyl (Z)-hexadec-9-enoate | C17H32O2 | 99 | - | 0.24 | - | 0.37 | 0.25 | |
| B2 | (E)-9-十六烯酸乙酯 Ethyl (E)-hexadec-9-enoate | C18H34O2 | 99 | - | - | - | 0.50 | 0.47 | |
| B3 | (Z)-13-十八烯酸甲酯 Methyl (Z)-octadec-13-enoate | C19H36O2 | 99 | 0.46 | - | - | - | - | |
| B4 | 棕榈酸甲酯 Methyl palmitate | C17H34O2 | 99 | 2.47 | 1.57 | 0.53 | 2.73 | 1.93 | |
| B5 | 棕榈酸乙酯 Ethyl palmitate | C18H36O2 | 99 | 0.70 | 0.23 | 0.34 | 2.79 | 2.28 | |
| B6 | 硬脂酸甲酯 Methyl stearate | C19H38O2 | 99 | 1.02 | 0.39 | - | 0.32 | 0.42 | |
| B7 | 硬脂酸乙酯 Ethyl stearate | C20H40O2 | 99 | 0.24 | - | - | 0.61 | 1.06 | |
| B8 | 反式油酸甲酯 Methyl (E)-octadec-9-enoate | C19H36O2 | 99 | - | - | - | 5.22 | 4.72 | |
| B9 | 油酸乙酯 Ethyl oleate | C20H38O2 | 99 | - | 0.53 | - | 9.76 | 6.03 | |
| B10 | 油酸甲酯 Methyl oleate | C19H36O2 | 99 | - | 4.29 | 0.53 | - | - | |
| B11 | 亚油酸甲酯 Methyl (9Z,12Z)-octadeca-9,12-dienoate | C19H34O2 | 99 | 0.27 | - | - | 1.61 | 1.80 | |
| B12 | 亚油酸乙酯 Ethyl linoleate | C20H36O2 | 99 | - | - | - | 2.36 | 2.30 | |
| B13 | 柠檬酸三乙酯 Triethyl citrate | C12H20O7 | 83 | - | - | - | 0.65 | - | |
| B14 | 苯酸丁酯 Butyl benzoate | C11H14O2 | 93 | 0.28 | - | - | - | - | |
| B15 | 肉豆蔻酸甲酯 Methyl tetradecanoate | C15H30O2 | 99 | 0.15 | - | - | - | - | |
| B16 | 肉豆蔻酸乙酯 Ethyl tetradecanoate | C16H32O2 | 94 | 0.03 | - | - | - | - | |
| B17 | 十五烷酸甲酯 Methyl pentadecanoate | C16H32O2 | 96 | 0.06 | - | - | - | - | |
| B18 | 邻苯二甲酸二仲丁酯 Di-sec-butyl phthalate | C16H22O4 | 91 | 0.14 | - | - | - | - | |
| B19 | 邻苯二甲酸二丁酯 Dibutyl phthalate | C16H22O4 | 97 | - | - | 4.67 | - | 1.60 | |
| B20 | 邻苯二甲酸二异丁酯 Diisobutyl phthalate | C20H30O4 | 90 | 0.18 | - | - | 0.57 | - | |
| B21 | 邻苯二甲酸单(2-乙基己基)酯 2-(((2-ethylhexyl)oxy) carbonyl) benzoic acid | C16H22O4 | 91 | - | 0.26 | - | 0.51 | - | |
| B22 | 对苯二甲酸二(2-乙基己)酯 Bis(2-ethylhexyl) terephthalate | C24H38O4 | 91 | - | - | 0.14 | - | - | |
| B23 | 丁基邻苯二甲酸环己酯 Butyl cyclohexyl phthalate | C18H24O4 | 90 | - | - | - | 3.80 | - | |
| B24 | 二(2-丙基戊基)邻苯二甲酸酯 Bis(2-propylpentyl) phthalate | C24H38O4 | 91 | - | - | 0.17 | - | - | |
| B25 | 二(2-乙基己基)邻苯二甲酸酯 Bis(2-ethylhexyl) phthalate | C24H38O4 | 98 | 2.72 | - | - | - | - | |
| B26 | (10E,13E)-十八碳二烯酸甲酯 Methyl (10E,13E)-octadeca-10,13-dienoate | C19H34O2 | 99 | - | 1.30 | - | - | - | |
| B27 | (11E,14E)-十八碳二烯酸甲酯 Methyl (11E,14E)-octadeca-11,14-dienoate | C19H34O2 | 99 | - | - | 0.43 | - | - | |
| B28 | 癸二酸二异辛酯 Dioctyl sebacate | C26H50O4 | 95 | 0.40 | - | 0.37 | - | 0.60 | |
| B29 | 己二酸二异辛酯 Bis(2-ethylhexyl) adipate | C22H42O4 | 95 | 8.57 | - | - | - | - | |
| B30 | 己二酸二甲酯 Dimethyl adipate | C8H14O4 | 91 | 0.11 | - | - | - | - | |
| B31 | 己二酸二(2-乙基己)酯 Bis(2-ethylhexyl) adipate | C22H42O4 | 94 | - | - | 0.39 | - | - | |
| B32 | 3-羟丙基油酸酯 3-hydroxypropyl oleate | C21H40O3 | 81 | - | - | - | 0.98 | - | |
| B33 | (E)-丙基-2,3-二羟基十八碳-9-烯酸酯 (E)-propyl 2,3-dihydroxyoctadec-9-enoate | C21H40O4 | 92 | - | - | 0.58 | - | - | |
| B34 | 2,3-二羟基棕榈酸丙酯 2,3-dihydroxypropyl oleate | C21H40O4 | 95 | - | - | 0.16 | - | 1.46 | |
| B35 | 8-((2R,3S)-3-辛基噁丙环-2-基)辛酸甲酯 Methyl 8-((2R,3S)-3-octyloxiran-2-yl) octanoate | C19H36O3 | 90 | 2.15 | - | - | - | - | |
| B36 | 反式-4-甲氧基肉桂酸异辛酯 2-ethylhexyl (E)-3-(4-methoxyphenyl) acrylate | C18H26O3 | 98 | 0.28 | - | - | - | - | |
| B37 | 18-甲基-十九烷酸甲酯 Methyl 18-methylnonadecanoate | C21H42O2 | 99 | 0.12 | - | - | - | - | |
| B38 | 8-(3-((3-戊基噁丙环-2-基)甲基)噁丙环-2-基)辛酸乙酯 ethyl 8-(3-((3-pentyloxiran-2-yl)methyl)oxiran-2-yl) octanoate | C20H36O4 | 83 | 1.04 | - | - | - | - | |
| B39 | B38的同分异构体 Isomer of B38 | C20H36O4 | 82 | 0.52 | - | - | - | - | |
| B40 | B38的同分异构体 Isomer of B38 | C20H36O4 | 82 | 0.12 | - | - | - | - | |
| 合计 Subtotal | 22.03 | 8.80 | 8.31 | 32.78 | 24.92 | ||||
| 醇类Alcohols | |||||||||
| C1 | (S)-(+)-甘油醇缩丙酮 (S)-(2,2-dimethyl-1,3-dioxolan-4-yl) methanol | C6H12O3 | 90 | - | - | 6.81 | - | - | |
| C2 | 1,2:5,6-二异亚丙基-D-甘露糖醇 1,2:5,6-bis-O-(1-methylethylidene)-D-mannitol | C12H22O6 | 91 | - | 0.72 | - | - | - | |
| C3 | 1,2:3,4:5,6-三异亚丙基-D-甘露糖醇 1,2:3,4:5,6-tri-O-(1-methylethylidene)-D-mannitol | C15H26O6 | 91 | - | - | 0.51 | - | - | |
| C4 | (3Z,13Z)-十八碳二烯-1-醇 (3Z,13Z)-octadeca-3,13-dien-1-ol | C18H34O | 91 | - | 0.62 | - | - | - | |
| C5 | Α-毕橙茄醇 (1R,4S,4aR,8aR)-4-isopropyl-1,6-dimethyl-1,2,3,4, 4a,7,8,8a-octahydronaphthalen-1-ol | C15H26O | 95 | 0.09 | - | - | - | - | |
| C6 | 1-十六烷醇(鲸蜡醇) Hexadecan-1-ol | C16H34O | 95 | 0.30 | - | - | - | - | |
| C7 | 二十二烷醇 Docosan-1-ol | C22H46O | 90 | 0.11 | - | - | - | - | |
| 合计Subtotal | 0.50 | 1.34 | 7.32 | - | - | ||||
| 酮类Ketones | |||||||||
| D1 | 4-甲基-3-戊烯-2-酮 4-methyl-3-pentene-2-one | C6H10O | 90 | - | - | - | - | 0.73 | |
| D2 | 4-甲氧基-4-甲基-2-戊酮 4-methoxy-4-methylpentan-2-one | C7H14O2 | 83 | - | - | - | - | 4.40 | |
| D3 | D2的同分异构体 Isomer of D2 | C7H14O2 | 82 | - | - | - | - | 0.10 | |
| D4 | 3,5-二甲基-2-环己烯-1-酮 3,5-dimethyl-2-cyclohexen-1-one | C8H12O | 80 | - | - | - | - | 0.33 | |
| D5 | 己烯-2-酮 3-hexen-2-one | C6H10O | 83 | - | 0.91 | - | - | - | |
| D6 | 二苯甲酮 Benzophenone | C13H10O | 95 | 0.41 | - | - | - | - | |
| 合计Subtotal | 0.41 | 0.91 | - | - | 5.56 | ||||
| 酚类Phenols | |||||||||
| E1 | 苯酚 Phenol | C6H6O | 83 | - | - | - | - | 1.00 | |
| E2 | 6,6′-亚甲基双-(2-叔丁基-4-甲基苯酚) 6,6′-methylenebis(2-(tert-butyl)-4-methylphenol) | C23H32O2 | 96 | - | 0.21 | - | - | - | |
| 合计 Subtotal | 0.21 | - | - | 1.00 | |||||
| 烷烃Alkanes | |||||||||
| F1 | 十一烷 Undecane | C11H24 | 94 | 0.02 | - | - | - | - | |
| F2 | 十二烷 Dodecane | C12H26 | 95 | 0.10 | - | - | - | - | |
| F3 | 十三烷 Tridecane | C13H28 | 86 | 0.02 | - | - | - | - | |
| F4 | 十五烷 Pentadecane | C15H32 | 94 | 0.10 | - | - | - | - | |
| F5 | 十六烷 Hexadecane | C16H34 | 93 | 0.06 | - | - | - | - | |
| F6 | 2,6,10,14-四甲基十五烷(降植烷) 2,6,10,14-tetramethylpentadecane (pristine) | C19H40 | 91 | 0.07 | - | - | - | - | |
| F7 | 十八烷 Octadecane | C18H38 | 93 | 0.06 | - | - | - | - | |
| F8 | 2,6,10,14-四甲基十六烷(植烷) 2,6,10,14-tetramethylhexadecane (phytane) | C20H42 | 83 | 0.04 | - | - | - | - | |
| 合计 Subtotal | 0.49 | - | - | - | - | ||||
| 烯烃Olefine | |||||||||
| G1 | 反式角鲨烯 (6E,10E,14E,18E)-2,6,10,15,19,23- hexamethyitetracosa-2,6,10,14,18,22-hexaene | C30H50 | 99 | 0.56 | - | - | - | - | |
| 合计 Subtotal | 0.56 | - | - | - | - | ||||
| 芳烃Aromatic hydrocarbon | |||||||||
| H1 | 1,2,3,4-四甲基苯 1,2,3,4-tetramethylbenzene | C10H14 | 93 | 0.03 | - | - | - | - | |
| H2 | 1,2,3,4,5-五甲基苯 1,2,3,4,5-pentamethylbenzene | C11H16 | 91 | 0.06 | - | - | - | - | |
| H3 | 2-甲基萘 2-methylnaphthalene | C11H10 | 94 | 0.20 | - | - | - | - | |
| H4 | 1-甲基萘 1-methylnaphthalene | C11H10 | 96 | 0.12 | - | - | - | - | |
| H5 | 2,7-二甲基萘 2,7-dimethylnaphthalene | C12H12 | 97 | 0.16 | - | - | - | - | |
| H6 | 1,3-二甲基萘 1,3-dimethylnaphthalene | C12H12 | 97 | 0.09 | - | - | - | - | |
| H7 | 3-甲基-1,1′-联苯基 3-methyl-1,1′-biphenyl | C13H12 | 92 | 0.05 | - | - | - | - | |
| H8 | 1-苯甲基-4-甲基苯 1-benzyl-4-methylbenzene | C14H14 | 95 | 0.08 | - | - | - | - | |
| H9 | 二苯基甲烷 Diphenylmethane | C13H12 | 83 | 0.05 | - | - | - | - | |
| 合计 Subtotal | 0.79 | - | - | - | - | ||||
| 杂原子Heteroatoms | |||||||||
| I1 | 芥酸酰胺 (Z)-docos-13-enamide | C22H43NO | 97 | - | - | - | 0.64 | 0.31 | |
| I2 | 油酸酰胺 oleamide | C18H35NO | 96 | - | 0.63 | - | 0.53 | - | |
| I3 | (4R,4′R)-2,2,2′,2′-四甲基-4,4′-二(1,3-二氧戊环) (4R,4′R)-2,2,2′,2′-tetramethyl-4,4′-bi(1,3-dioxolane) | C10H18O4 | 91 | - | 0.39 | - | - | - | |
| I4 | (4R,4′R,4′′R,5′R)-2,2,2′,2′, 2′′,2′′-六甲基-4,4′:5′,4′′-三(1,3-二氧戊环) (4R,4′R,4′′R,5′R)-2,2,2′,2′, 2′′,2′′-hexamethyl-4,4′:5′,4′′-ter(1,3-dioxolane) | C15H26O6 | 91 | - | - | 0.24 | - | - | |
| I5 | 1,1-二甲氧基壬烷 1,1-dimethoxynonane | C11H24O2 | 83 | - | 0.46 | - | - | - | |
| I6 | 1-乙基-2-甲基二硫烷 1-ethyl-2-methyldisulfane | C3H8S2 | 91 | 0.04 | - | - | - | - | |
| I7 | 1-甲基-2-丙基二硫烷 1-methyl-2-propyldisulfane | C4H10S2 | 86 | 0.01 | - | - | - | - | |
| I8 | 1,2-二乙基二硫烷 1,2-diethyldisulfane | C4H10S2 | 90 | 0.06 | - | - | - | - | |
| I9 | 1-乙基-2-异丙基二硫烷 1-ethyl-2-isopropyldisulfane | C5H12S2 | 87 | 0.03 | - | - | - | - | |
| 合计 Subtotal | 0.14 | 1.48 | 0.24 | 1.17 | 0.31 | ||||
| 甾类Steroids | |||||||||
| J1 | (22E,24R)-麦角甾-5,8,22-三烯-3beta-醇 (22E,24R)-ergosta-5,8,22-triene-3beta-ol | C28H44O | 91 | - | - | 0.25 | - | 2.06 | |
| J2 | 7,22-麦角二烷醇 7,22-ergostadienol | C28H46O | 99 | - | - | - | - | 0.92 | |
| J3 | 7-麦角甾烯醇 7-ergostenol | C28H48O | 91 | - | - | - | - | 0.37 | |
| J4 | 7,22-麦角甾二烯酮 7,22-ergostadienone | C28H44O | 81 | 0.15 | - | - | - | - | |
| J5 | 5,6-二氢麦角甾醇 5,6-dihydroergosterol | C28H46O | 99 | 0.21 | 0.38 | 0.14 | 0.41 | - | |
| J6 | 麦角甾-4,6,8(14),22-四烯-3-酮 Ergosta-4,6,8(14),22-tetraen-3-one | C28H40O | 95 | 0.86 | - | - | - | 0.33 | |
| J7 | 3,5-环-6,8(14),22-麦角三烯 3,5-cyclo-6,8(14),22-ergostatriene | C28H42 | 87 | - | - | 0.04 | - | - | |
| J8 | 新麦角甾醇 Neoergosterol | C27H40O | 99 | 0.16 | - | 0.04 | - | - | |
| J9 | 胆固醇 Cholesterol | C27H46O | 99 | 0.21 | - | - | - | - | |
| 合计Subtotal | 1.59 | 0.38 | 0.47 | 0.41 | 3.68 | ||||
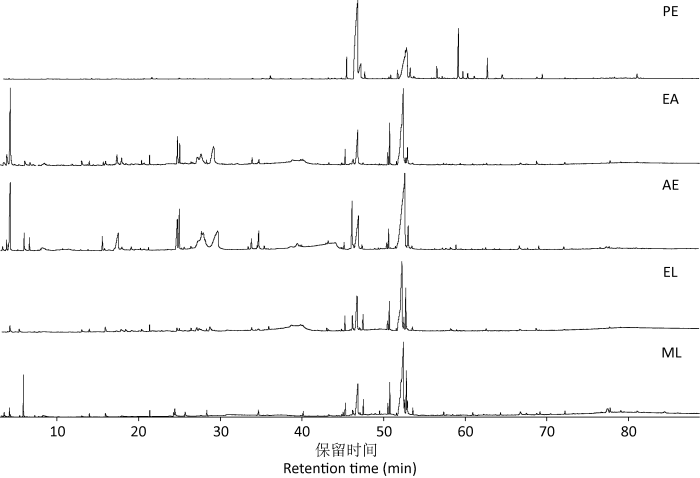
图1
图1
灵芝孢子粉5级萃取物的GC-MS总离子流色谱图
Fig. 1
Total ion chromatograms by GC-MS analysis of the extracts from Ganoderma lingzhi spore powder using petroleum ether (PE), ethyl acetate (EA), acetone (AE), ethanol (EL) and methanol (ML) as solvents.
2.1.1 各萃取物中主要化合物及含量组成:石油醚萃取物PE中鉴定出57种化合物,其中相对含量较高为油酸(25.61%)、己二酸二异辛酯(8.57%)和棕榈酸(4.10%);乙酸乙酯萃取物EA中鉴定出22种,相对含量较高为(E)-6-十八碳-烯酸(36.47%)、棕榈酸(9.61%)和油酸甲酯(4.29%);丙酮萃取物AE中鉴定出23种,相对含量较高为(E)-9-十八碳烯酸(33.30%)、(S)-(+)-甘油醇缩丙酮(6.81%)和邻苯二甲酸二丁酯(4.67%);乙醇萃取物EL中鉴定出20种,相对含量较高为(E)-十八碳-6-烯酸(40.01%)、棕榈酸(13.49%)、油酸乙酯(9.76%)和反式油酸甲酯(5.22%);甲醇萃取物ML中鉴定出28 种,其中(Z)-6-十八碳-烯酸(26.24%)、亚油酸(17.34%)、棕榈酸(12.52%)、油酸乙酯(6.03%)、反式油酸甲酯(4.72%)和4-甲氧基- 4-甲基-2-戊酮(4.46%)的相对含量较高(表1)。总体来看,从灵芝孢子粉5种萃取物中共鉴定出101种化合物。
2.1.2 各萃取物中化合物的类型及构成:从灵芝孢子粉5种萃取物中鉴定出的化合物有8类,包括酸类、酯类、醇类、酮类、酚类、烃类(含烷烃、烯烃和芳烃类)、杂原子类和甾类(表1)。
5种萃取物中酸类化合物共有10种,萃取物EA、ML、PE、AE和EL中酸类化合物分别为6种、5种、4种、4种和2种,依次减少;总体来看,萃取物中酸类物质的含量较高,5种萃取物中酸类物质的相对含量均值接近46.3%,其中甲醇萃取物ML中酸类化合物的相对含量高达58.58%,尽管乙醇萃取物EL中仅有2种酸类化合物,但其相对含量达到53.50%,乙酸乙酯萃取物EA中酸类化合物也高达50.48%,相对而言,丙酮萃取物AE和石油醚萃取物PE中酸类化合物的相对含量有所减少,但也超过30%,分别为36.24%和31.87%。由此可见,在灵芝孢子粉中酸类化合物的种类数虽然只有10种,但其相对含量却较高。
5种萃取物中酯类化合物共有40种,其中PE、EA、AE、EL和ML中分别为22种、8种、11种、15种和13种,相对含量分别为22.03%、8.80%、8.29%、32.77%和24.92%。与酸类物质相比,灵芝孢子粉萃取物中酯类化合物的种数明显增多,但相对含量显著下降。郭志峰等(2010)利用GC/MS分析了破壁灵芝孢子粉乙醇提取液的乙醚萃取物,共分离出39个峰,鉴定出24种化合物,其中酯类物质有12种,主要为C4-C10的小分子量化合物,而本研究通过索氏提取方法有效分离并鉴定酯类化合物达40种,主要为C10-C26的相对较大分子量化合物,除EA和AE中酯类化合物为8种和11种外,其他3种萃取物中均多于12种,表现出不同溶剂对酯类物质的差异性分离。两实验鉴定出的酯类化合物数量差异较大,这可能与索氏分级萃取法较单一溶剂提取法对灵芝孢子粉中酯类物质的分离更充分有关,当然两实验中灵芝样本的差异也会影响鉴定结果。
5种萃取物中醇类化合物共有7种,其中在PE、EA和AE醇类化合物分别有3种、2种和2种,相对含量分别为0.50%、1.34%和7.32%,而EL和ML中未鉴定出醇类物质。
5种萃取物中酮类化合物共有6种,其中PE和EA均只有1种,相对含量分别为0.41%和0.91%;ML中最多有4种,相对含量为5.56%;而AE和EL中未鉴定出酮类物质。从5种溶剂对灵芝孢子粉中酮类物质分离效果上来看,甲醇萃取物中的酮类化合物种类最多,较为适合灵芝孢子粉中酮类物质的分离与萃取。
5种萃取物中酚类化合物共鉴定出2种,其中EA和ML中各有1 种,相对含量分别为0.21%和1.00%。烃类化合物仅在PE中共鉴定出18种,相对含量为1.84%。相对于其他萃取剂,石油醚对烃类物质的分离效果明显,这可能与烃类化合物极性小,按照相似相容原理,其易于被极性较小的石油醚溶剂分离和萃取所致。
5种萃取物中杂原子类物质共有9种,其中PE、EA、AE、EL和ML中分别有4种、3种、2种、2种和1种,相对含量分别为0.14%、1.48%、0.24%、1.17%和0.31%。
5种萃取物中甾类物质共有15种。其中PE有5种,AE和ML均有4种,EA和EL均为1种,总体来看,除ML中甾类物质相对含量为3.68%外,其他4种溶剂的萃取物中甾类物质的相对含量都不高。这可能是因为甾类化合物分子中都含有一个叫甾核的四环碳骨架,而环上的3号碳原子上通常为羟基或羰基,易与甲醇形成氢键有关。
2.2 UPLC-Q-TOF/MS分析
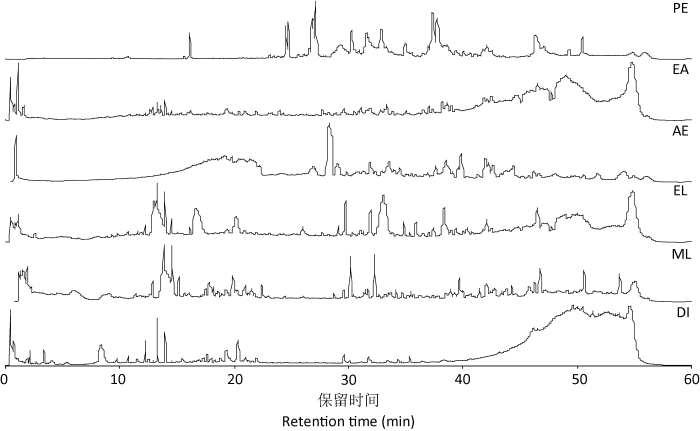
图2
图2
灵芝孢子粉6种级萃取物的UPLC-Q-TOF/MS全扫描总离子流图
Fig. 2
Total ion chromatograms of the extracts from Ganoderma lingzhi spore powder using petroleum ether (PE), ethyl acetate (EA), acetone (AE), ethanol (EL), methanol (ML) and deionized water (DI) as solvents analysed by UPLC-Q-TOF/MS.
表2 灵芝孢子粉6级萃取物中化合物的UPLC-Q-TOF/MS鉴定结果
Table 2
| 分类 Type | (min) | [M+H]+ | 碎片离子 Fragment ion | 误差 Error (10-3) | 分子式 Molecular formula | 化合物 Chemical compound | 来源 Resources | ||
|---|---|---|---|---|---|---|---|---|---|
| 实测值 Measured value | 理论值 Theoretical value | ||||||||
| 萜类 Terpenes | |||||||||
| 1 | 17.68 | 514.2930 | 514.2929 | 497.2902, 479.2794, 498.2937 | 0.1 | C30H42O7 | 灵芝酸A Ganoderenic acid A | DI | |
| 2 | 18.78 | 530.2886 | 530.2867 | 473.2543, 513.2828, 497.2901 | 1.9 | C30H42O8 | 灵芝酸C6 Ganoderenic acid C6 | DI | |
| 3 | 21.94 | 570.2838 | 570.2829 | 515.3002 | 0.9 | C32H42O9 | 灵芝酸F Ganoderenic acid F | EA | |
| 4 | 19.33 | 572.2985 | 572.2984 | 499.3062, 481.2953 | 0.1 | C32H44O9 | 灵芝酸H Ganoderenic acid H | DI | |
| 5 | 17.36 | 516.2727 | 516.2723 | 444.2829, 443.2800, 425.2687 | 0.4 | C29H40O8 | 赤芝酸E Lucidenic acid E | DI | |
| 6 | 40.12 | 408.3027 | 408.3025 | 393.3145, 224.1300 | 0.2 | C28H40O2 | 2,7,8-三甲基-2-((3E,7E)-4,8,12-三甲基十三碳-3,7,11- 三烯-1-基)-2H-苯并吡喃 2,7,8-trimethyl-2-((3E,7E)-4,8,12-trimethyltrideca-3,7,11- trien-1-yl)-2H-chromen-6-ol | PE | |
| 7 | 20.48 | 496.2827 | 496.2825 | 479.2795 | 0.2 | C30H40O6 | (2R,4aS,6aR,12bR,14aS)-9-甲酰基-10,11-二羟基-2,4a,6a, 12b,14a-五甲基-8氧代-1,2,3,4,4a,5,6,6a,6b,7,8,12b,13, 14,14a,14b-十六氢苉-2-羧酸甲酯 Methyl (2R,4aS,6aR,12bR,14aS)-9-formyl-10,11-dihydroxy- 2,4a,6a,12b,14a-pentamethyl-8-oxo-1,2,3,4,4a,5,6,6a,6b,7, 8,12b,13,14,14a,14b-hexadecahydropicene-2-carboxylate | EA | |
| 8 | 29.18 | 519.3327 | 519.3305 | 467.3222, 460.3157, 459.3097 | 2.2 | C33H45NO4 | (4aS,6aR,6bS,8aR,12aS)-11-氰基-2,2,6a,6b,9,9,12a- 七甲基-10,14-二氧代-1,3,4,5,6,6a,6b,7,8,8a,9,10,12a,14, 14a,14b-十六氢苉-4a(2H)-羧酸乙酯 Ethyl (4aS,6aR,6bS,8aR,12aS)-11-cyano-2,2,6a,6b,9,9,12a- heptamethyl-10,14-dioxo-1,3,4,5,6,6a,6b,7,8,8a,9,10,12a,14, 14a,14b-hexadecahydropicene-4a(2H)-carboxylate | ML, EL | |
| 9 | 46.12 | 456.3236 | 456.3232 | 330.3006, 319.3042, 318.3010 | 0.4 | C29H44O4 | (4aR,5R,6aS,6bR,8aS,9R,12aS,14bS)-5-羟基-2,2,6a,6b,9,12a- 六甲基-10-氧代-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a, 12b,13,14b-二十氢苉-4a-羧酸 (4aR,5R,6aS,6bR,8aS,9R,12aS,14bS)-5-hydroxy-2,2,6a,6b,9, 12a-hexamethyl-10-oxo-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11, 12,12a,12b,13,14b-icosahydropicene-4a-carboxylic acid | ML | |
| 10 | 19.40 | 572.2988 | 572.2986 | 513.2844, 481.2950, 463.2846 | 0.2 | C32H44O9 | (Z)-6-((3S,5R,7S,10S,12S,13R,14R,17R)-12-乙酰氧基--3,7-二羟基- 4,4,10,13,14-五甲基-11,15-二氧代-2,3,4,5,6,7,10,11,12,13,14,15, 16,17-十四氢-1H-环戊烯并[a]菲-17-基)-2-甲基-4-氧代庚-5-烯酸 (Z)-6-((3S,5R,7S,10S,12S,13R,14R,17R)-12-acetoxy-3,7-dihydroxy- 4,4,10,13,14-pentamethyl-11,15-dioxo-2,3,4,5,6,7,10,11,12,13,14, 15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)- 2-methyl-4-oxohept-5-enoic acid | EA, AE, EL | |
| 11 | 26.68 | 498.2989 | 498.2982 | 423.2813, 397.2463, 365.2214 | 0.7 | C30H42O6 | (3E,5E)-(1aR,1bS,4aR,7aS,7bR,8S,9aS)-4a,7b-二羟基-3- (羟甲基)-1,1,6,8-四甲基-5-氧代-1a,1b,4,4a,5,7a,7b,8,9,9a- 十氢-1H-环丙[3,4]苯并[1,2-e]甘菊环-9a-基十碳-3,5-二烯酸酯 (3E,5E)-(1aR,1bS,4aR,7aS,7bR,8S,9aS)-4a,7b-dihydroxy-3- (hydroxymethyl)-1,1,6,8-tetramethyl-5-oxo-1a,1b,4,4a,5,7a,7b,8,9,9a- decahydro-1H-cyclopropa[3,4]benzo[1,2-e]azulen-9a-yl deca-3,5-dienoate | AE | |
| 生物碱类Alkaloids | |||||||||
| 12 | 16.67 | 197.1535 | 197.1526 | 171.1470 | 0.9 | C10H19N3O | 5-氨基-1-(庚烷-2-基)-1,2-二氢-3H-吡唑-3-酮 5-amino-1-(heptan-2-yl)-1,2-dihydro-3H-pyrazol-3-one | EL | |
| 13 | 26.02 | 287.2467 | 287.2461 | 124.0851, 118.0875, 114.0911 | 0.6 | C16H33NO3 | (2S,3S,4S,5S)-2-(羟甲基)-5-十一基吡咯烷-3,4-二醇 (2S,3S,4S,5S)-2-(hydroxymethyl)-5-undecylpyrrolidine-3,4-diol | EL | |
| 14 | 8.43 | 169.1219 | 169.1215 | 152.1188 | 0.4 | C8H15N3O | 4-氨基-3-戊基-1H-吡唑-5-醇 4-amino-3-pentyl-1H-pyrazol-5-ol | DI | |
| 15 | 31.94 | 521.3483 | 521.3461 | 498.3794, 480.3081 | 2.2 | C33H47NO4 | (4R,4aS,6R,7R,7aR,12bS)-6-((S)-4-环己基-2-羟基丁烷-2-基)-3- (环丙基甲基)-7-甲氧基-1,2,3,4,5,6,7,7a-八氢-4a,7-乙桥- 4,12-甲桥苯并呋喃[3,2-e]异喹啉-9-醇 (4R,4aS,6R,7R,7aR,12bS)-6-((S)-4-cyclohexyl-2-hydroxybutan-2-yl)- 3-(cyclopropylmethyl)-7-methoxy-1,2,3,4,5,6,7,7a-octahydro-4a,7- ethano-4,12-methanobenzofuro[3,2-e]isoquinolin-9-ol | ML | |
| 酰胺类Amides | |||||||||
| 16 | 38.40 | 281.2731 | 281.2719 | 265.2542 | 1.2 | C18H35NO | 油酸酰胺 Oleamide | AE | |
| 17 | 35.37 | 279.2565 | 279.2562 | 263.2380, 245.2265 | 0.3 | C18H33NO | 亚油酰胺 (9Z,12Z)-octadeca-9,12-dienamide | EL | |
| 18 | 34.85 | 343.3093 | 343.3087 | 199.1796, 194.1154, 178.1249 | 0.6 | C20H41NO3 | N-((2S,3R)-1,3-二羟基十八烷-2-基)乙酰胺 N-((2S,3R)-1,3-dihydroxyoctadecan-2-yl)acetamide | EL | |
| 19 | 35.94 | 369.3244 | 369.3241 | 342.3002, 280.2392, 279.2337 | 0.3 | C22H43NO3 | N-((4S,5R,E)-1,5-二羟基二十碳-6-烯-4-基)乙酰胺 N-((4S,5R,E)-1,5-dihydroxyicos-6-en-4-yl)acetamide | EL | |
| 20 | 39.43 | 371.2403 | 371.2400 | 354.3362, 282.2781, 178.1221 | 0.3 | C22H45NO3 | N-((4S,5R)-1,5-二羟基二十烷-4-基)乙酰胺 N-((4S,5R)-1,5-dihydroxyicosan-4-yl)acetamide | EL | |
| 21 | 50.49 | 392.3086 | 392.3039 | 268.1835, 253.1600 | 4.7 | C23H40N2O3 | (2R,4S,5S)-5-氨基-N-丁基-4-羟基-9-(3-(羟甲基)苯基)- 2,7,7-三甲基壬酰胺 (2R,4S,5S)-5-amino-N-butyl-4-hydroxy-9-(3-(hydroxymethyl) phenyl)-2,7,7-trimethylnonanamide | ML | |
| 22 | 33.37 | 473.3707 | 473.3684 | 458.3467, 341.2063 | 2.3 | C27H47N5O2 | (R)-2-((2R,5S)-5-((R)-2-(4-(3-(二丁基氨基)丙-1-炔-1-基)-1H- 1,2,3-三唑-1-基)丙基)四氢呋喃-2-基)-N,N-二乙基丙酰胺 (R)-2-((2R,5S)-5-((R)-2-(4-(3-(dibutylamino)prop-1-yn-1-yl)-1H-1,2,3- triazol-1-yl)propyl)tetrahydrofuran-2-yl)-N,N-diethylpropanamide | AE | |
| 酸类Acids | |||||||||
| 23 | 30.32 | 358.2671 | 358.2669 | 293.2095, 279.2316, 263.2316 | 0.2 | C20H38O5 | 前列腺素F0α prostaglandin F0α | PE | |
| 24 | 31.94 | 294.2199 | 294.2195 | 278.2204, 277.2172, 118.0876 | 0.4 | C18H30O3 | 13-羟基十八碳-6,9,11-三烯酸 13-hydroxyoctadeca-6,9,11-trienoic acid | EA,DI | |
| 25 | 38.25 | 310.2517 | 310.2508 | 279.2329, 118.0877 | 0.9 | C19H34O3 | 14-羟基十九碳-10,12-二烯酸 14-hydroxynonadeca-10,12-dienoic acid | EA | |
| 26 | 12.93 | 242.0810 | 242.0791 | 198.0671, 172.0877 | 1.9 | C11H14O6 | 2-(3,4,5-三甲氧基苯氧基)乙酸 2-(3,4,5-trimethoxyphenoxy)acetic acid | DI | |
| 27 | 32.81 | 296.2354 | 296.2352 | 279.2324, 169.1199, 124.0890 | 0.2 | C18H32O3 | (9E,11Z)-13-羟基十八碳-9,11-二烯酸 (9E,11Z)-13-hydroxyoctadeca-9,11-dienoic acid | EA | |
| 28 | 27.21 | 338.2438 | 338.2419 | 311.2191, 293.2101, 281.2459 | 1.9 | C20H34O4 | (5Z,8Z,14E)-11,12-二羟基二十碳-5,8,14-三烯酸 (5Z,8Z,14E)-11,12-dihydroxyicosa-5,8,14-trienoic acid | EA | |
| 29 | 36.52 | 308.2357 | 308.2352 | 279.2328, 118.0874 | 0.5 | C19H32O3 | (S,9Z,11E)-13-甲氧基十八碳-6,9,11-三烯酸 (S,9Z,11E)-13-methoxyoctadeca-6,9,11-trienoic acid | EA,EL | |
| 30 | 39.02 | 310.2515 | 310.2508 | 279.2329, 124.0876, 118.0874 | 0.7 | C19H34O3 | (S,9Z,11E)-13-甲氧基十八碳-9,11-二烯酸 (S,9Z,11E)-13-methoxyoctadeca-9,11-dienoic acid | EL | |
| 31 | 40.47 | 324.2667 | 324.2665 | 279.2325, 124.0867 | 0.2 | C20H36O3 | (Z)-16-((2R,3S)-3-乙基环氧乙烷-2-基)十六烷-14-烯酸 (Z)-16-((2R,3S)-3-ethyloxiran-2-yl)hexadec-14-enoic acid | EL | |
| 酯类Esters | |||||||||
| 32 | 16.12 | 276.1217 | 276.1215 | 154.9904, 141.9812, 112.9787 | 1.2 | C12H20O7 | (2R,3S,4R)-3,4-二羟基-5-氧代四氢呋喃-2-基碳酸庚酯 (2R,3S,4R)-3,4-dihydroxy-5-oxotetrahydrofuran-2-yl heptyl carbonate | PE, EL | |
| 33 | 24.78 | 344.2569 | 344.2563 | 295.2262, 279.2335, 277.2148 | 0.6 | C19H36O5 | (5R)-5-((1R,4R)-1,4,5-三羟基十五烷基)二氢呋喃-2(3H)-酮 (5R)-5-((1R,4R)-1,4,5-trihydroxypentadecyl)dihydrofuran-2(3H)-one | PE | |
| 34 | 26.79 | 390.2982 | 390.2960 | 381.2612, 355.2808, 354.2840 | 2.2 | C23H41O6 | (Z)-7-((1R,3R,5S)-3,5-二羟基-2-((3S,4S,E)-3-羟基-4-甲氧基-4- 甲基辛-1-烯-1-基)环戊基)庚-5-烯酸甲酯 Methyl (Z)-7-((1R,3R,5S)-3,5-dihydroxy-2-((3S,4S,E)-3-hydroxy-4- methoxy-4-methyloct-1-en-1-yl)cyclopentyl)hept-5-enoate | PE | |
| 醇类Alcohols | |||||||||
| 35 | 16.94 | 500.3138 | 500.3137 | 483.3101, 465.2975, 459.2707 | 0.1 | C30H44O6 | (E)-5-(4-(十六烷基氧代)-3,5-二羟基苯乙烯基)苯-1,2,3-三醇 (E)-5-(4-(hexadecyloxy)-3,5-dihydroxystyryl)benzene-1,2,3-triol | ML | |
| 36 | 41.19 | 400.2879 | 400.2872 | 124.0875, 118.0876 | 0.7 | C28H38O2 | (S)-3-(2-((3aS,7aS)-3-(1-(4-羟基-4-甲基戊-2-氰基-1-基)环丙基)-3a- 甲基-3a,4,5,6-四氢-1H-茚-7(7aH)-亚基)亚乙基)-2-亚甲基环己醇 (S)-3-(2-((3aS,7aS)-3-(1-(4-hydroxy-4-methylpent-2-yn-1-yl) cyclopropyl)-3a-methyl-3a,4,5,6-tetrahydro-1H-inden-7(7aH)- ylidene)ethylidene)-2-methylenecyclohexanol | ML | |
| 37 | 24.01 | 484.2065 | 484.2033 | 470.2393, 429.2488, 365.2303 | 3.2 | C27H32O8 | (3S,4R,6S,6aS,12bS)-3,6-二羟基-4-(羟甲基)-9-(4-甲氧基苯基)- 4,6a,12b-三甲基-1,3,4,4a,5,6,6a,12b-八氢苯并[f]吡喃酮[4,3-b] 苯并吡喃-11,12(2H,12aH)-二酮 (3S,4R,6S,6aS,12bS)-3,6-dihydroxy-4-(hydroxymethyl)-9-(4- methoxyphenyl)-4,6a,12b-trimethyl-1,3,4,4a,5,6,6a,12b- octahydrobenzo[f]pyrano[4,3-b]chromene-11,12(2H,12aH)-dione | EA | |
| 糖类Saccharides | |||||||||
| 38 | 34.30 | 499.3870 | 499.3867 | 447.3879, 425.3058 | 0.3 | C28H53NO6 | 2-(十四基(((3aR,5R,5aS,8aS,8bR)-2,2,7,7-四甲基四氢-3aH-二 ([1,3]二氧)[4,5-b:4',5'-d]吡喃-5-基)甲基)氨基)乙醇 2-(tetradecyl(((3aR,5R,5aS,8aS,8bR)-2,2,7,7-tetramethyltetrahydro- 3aH-bis([1,3]dioxolo)[4,5-b:4',5'-d]pyran-5-yl)methyl)amino)ethanol | ML | |
| 糖苷类Glycosides | |||||||||
| 39 | 27.76 | 490.3878 | 490.3870 | 297.2426, 279.2321, 224.1285 | 0.8 | C27H54O7 | β-6-脱氧-D-六碳吡喃糖鲨肝醇苷 Lochmodoside | EA | |
| 甾类Steroids | |||||||||
| 40 | 31.94 | 521.3478 | 521.3451 | 354.2852, 318.2122 | 2.7 | C33H47NO4 | (3S,8R,9S,10R,13S,14S,17S,E)-10,13-二甲基-3-(吡咯烷-1-基)- 16-(3,4,5-三甲基苯亚甲基)-2,3,4,7,8,9,10,11,12,13,14,15,16,17- 十四氢-1H-环戊烯并[a]菲-17-醇 (3S,8R,9S,10R,13S,14S,17S,E)-10,13-dimethyl-3-(pyrrolidin-1-yl)-16- (3,4,5-trimethoxybenzylidene)-2,3,4,7,8,9,10,11,12,13,14,15,16,17- tetradecahydro-1H-cyclopenta[a]phenanthren-17-ol | EL | |
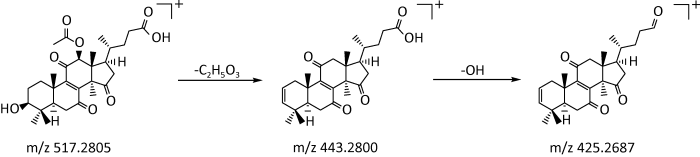
2.2.1 萜类:萜类化合物是灵芝等天然产物中数量较多的一类化合物,分布极为广泛(Baby et al. 2015;谭贻等 2019)。根据分子骨架中异戊二烯单元的数目可以分为单萜、倍半萜、二萜、三萜等(Kirby & Keasling 2009;徐硕和卢文玉 2019)。本研究从6种溶剂萃取物中共鉴定出了11种萜类成分,其中三萜类9种,二萜类1种,倍半萜类1种。就6种溶剂萃取物中的萜类而言,PE中有二萜类化合物1种(即化合物6);EA中有三萜类化合物3种(即化合物3、7和10);AE中三萜类化合物1种(即化合物10)和倍半萜类化合物1种(即化合物11);EL中三萜类化合物2种(即化合物8和10);ML中三萜类化合物2种(即化合物8和9);DI中三萜类化合物4种(即化合物1、2、4和5)。在EA、AE和EL等3种萃取物中均有三萜类化合物10,EL和ML中均有三萜类化合物8。萜类化合物在正离子模式下响应较好,在质谱条件下主要通过丢失H2O、OH、CH3COOH等侧链基团而形成碎片离子,母核的环状结构一般不会发生裂解。以三萜类化合物5为例,在正离子模式下,其保留时间为17.36min,利用Masslynx软件中的Massfragment功能得到准离子峰m/z517.2805[M+H]+,其二级碎片离子主要有m/z444.2829[M+H-C2H4O3]+、m/z443.2800[M+ H-C2H5O3]+和m/z425.2687[M+H-C2H6O4]+。根据数据库匹配出分子式为C29H40O8,分子量为516.2725,推测该化合物可能为赤芝酸E,可能的裂解途径见图3。
图3
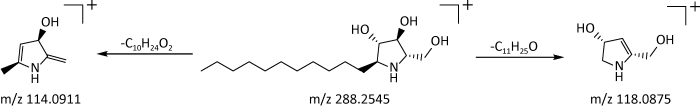
2.2.2 生物碱类:真菌生物碱是真菌中的一类重要代谢产物,一直备受国内外学者的关注(路莉文等 2018)。本研究从6种萃取物中共鉴定出了4种生物碱成分,包括吡唑类2个、吡咯类1个、异喹啉类1个。其中,在EL中鉴定出1种吡唑类化合物(即化合物12)和1种吡咯类化合物(即化合物13);ML中有1种异喹啉类化合物(即化合物15);DI中有1种吡唑类化合物(即化合物14)。生物碱类化合物在正离子模式下响应也较好,在质谱条件下主要是失去H2O、烷基等中性侧链基团。以化合物13为例,在正离子模式下,其保留时间为26.02min,利用Masslynx软件中的Massfragment功能获得准离子峰m/z288.2545 [M+H]+,二级碎片离子主要有m/z118.0875[M+H- C11H25O]+、m/z114.0911[M+H-C10H24O2]+。根据数据库匹配出分子式为C16H33NO3,分子量为287.2465,推测该化合物可能为(2S,3S,4S,5S)-2-(羟甲基)-5-十一基吡咯烷-3,4-二醇,可能裂解途径见图4。
图4
图4
(2S,3S,4S,5S)-2-(羟甲基)-5-十一基吡咯烷-3,4-二醇可能的裂解途径
Fig. 4
Possible bond-breaking pathways of (2S,3S,4S,5S)-2-(hydroxymethyl)-5-undecylpyrrolidine-3,4-diol.
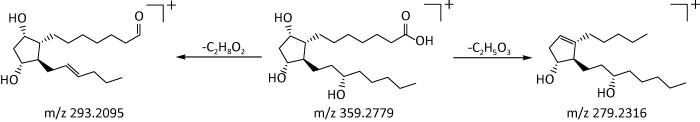
2.2.3 有机酸类:有机酸分子中含有羧酸,要包括脂肪族、芳香族和萜类3类,具有抗炎症、抗氧化、抑制血小板聚集的药理作用,并对心血管疾病的防治具有重要的临床应用价值(汤喜兰等2012)。本研究从6种萃取物中鉴定出了9种有机酸成分,主要为不饱和脂肪酸。其中,从PE中鉴定出1种(即化合物23);EA中5种(即化合物24、25、27、28和29);EL中3种(化合物29、30和31);DI中2种(即化合物24和26)。化合物24是EA和DI的共有化合物,化合物29是EA和EL的共有化合物。有机酸类化合物在正离子模式下响应较好,在质谱条件下的裂解规律主要是失去H2O、OH等中性碎片。以化合物23为例,在正离子模式下,其保留时间为30.32min,利用Masslynx软件中的Massfragment功能得到准离子峰m/z 359.2779[M+H]+,其二级碎片离子主要有m/z293.2095[M+H-C2H8O2]+、m/z279.2316 [M+H-C2H5O3]+、m/z263.2316[M+H-C2H6O4]+。根据数据库匹配出分子式为C20H38O5,分子量为358.2699,推测该化合物可能为前列腺素F0α,可能的裂解途径见图5。
图5
图5
前列腺素F0α可能的裂解途径
Fig. 5
Possible bond-breaking pathways of prostaglandin F0α.
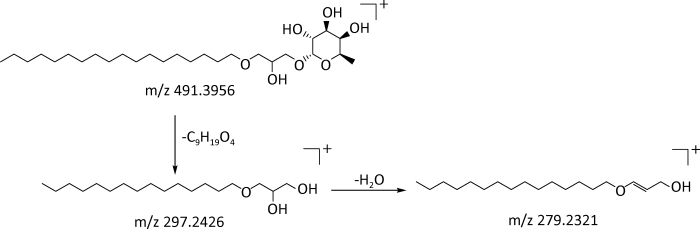
2.2.4 其他类:其他类主要包含糖类、糖苷类、酯类、醇类和甾类等化合物。糖苷类化合物在正离子模式下响应较好,在质谱条件下主要易失去糖分子。以化合物39为例,在正离子模式下,其保留时间为27.76min,利用Masslynx软件中的Massfragment功能得到准离子峰m/z491.3956 [M+H]+,其二级碎片离子主要有m/z297.2426[M+ H-C9H19O4]+、m/z279.2321[M+H-C9H20O5]+。根据数据库匹配出分子式为C27H54O7,分子量为490.3876,推测该化合物可能为β-6-脱氧-D-六碳吡喃糖鲨肝醇苷,可能的裂解途径见图6。
图6
图6
β-6-脱氧-D-六碳吡喃糖鲨肝醇苷可能的裂解途径
Fig. 6
Possible bond-breaking pathways of lochmodoside.
3 讨论
灵芝孢子粉中次生代谢产物的化学组成复杂,分子间的极性和分子量差别较大,传统的萃取分离方法和单一的检测技术均在一定程度上限制了样品成分的分离与鉴定。本研究采用了极性递增的6种溶剂(石油醚、乙酸乙酯、丙酮、乙醇、甲醇和水)依次对灵芝孢子粉中的化学物质进行索氏提取,以达到样品中次生代谢产物按极性差异实现分离的目的,同时根据GC/MS方法和UPLC-Q-TOF/MS技术在上机样品的处理要求、适宜检测的分子量阈以及检测的灵敏度等方面的差异(邹海洋等 2010;Lacina et al. 2017),利用GC/MS和UPLC-Q-TOF/MS两种技术同步检测萃取物样品,以获取更大范围的次生代谢产物的分析与鉴定。本研究从实验样品灵芝孢子粉萃取物中共鉴定出140种化合物,其中利用GC/MS技术从5种溶剂(除水)萃取物中共鉴定出101种化合物,利用UPLC-Q-TOF/MS技术从6种溶剂中共鉴定出40种化合物,两种检测技术重复鉴定出的化合物仅有油酸酰胺,显示出两种检测技术在次生代谢产物检测范围上具有明显的差异。就GC/MS的检测结果而言,各级萃取物均以酸类和酯类化合物为主,但含量存在差异,并表现出一定的变化特征。如甲醇、乙醇萃取物中的酯类化合物含量相对较高;这可能与有机酸的性质有关,酸类化合物的热稳定性一般较差,易与甲醇、乙醇发生酯化反应生成酯类化合物(甘卓亭等 2019),从而导致酯类物质含量增高。在石油醚萃取物中酸类化合物的相对含量最低,为31.87%;除丙酮萃取物外,随着溶剂极性的增大酸类化合物的相对含量总体呈递增趋势。
就UPLC-Q-TOF/MS检测结果而言,在鉴定和推测出40种化合物中有9种为三萜类化合物。灵芝三萜类化合物以四环三萜和五环三萜为主,本研究鉴定出四环三萜类有6种,分别为灵芝酸A、灵芝酸C6、灵芝酸F、灵芝酸H、赤芝酸E和化合物10;五环三萜类有2种,分别为化合物8和化合物9。灵芝三萜类物质一般难溶于水,易溶于甲醇、乙醇、乙酸乙酯等有机溶剂,但本研究水提取物中三萜类物质最多,乙酸乙酯萃取物次之,这可能与萃取方式有关。当灵芝孢子粉经5种有机溶剂逐级提取后,其内部结构已趋于疏松,待后续水提时三萜类物质就较易于被溶解与吸附;另外,索氏提取过程中需加热抽提,萜类物质热运动加快,也在一定程度上促进了水溶剂对三萜类物质的提取。
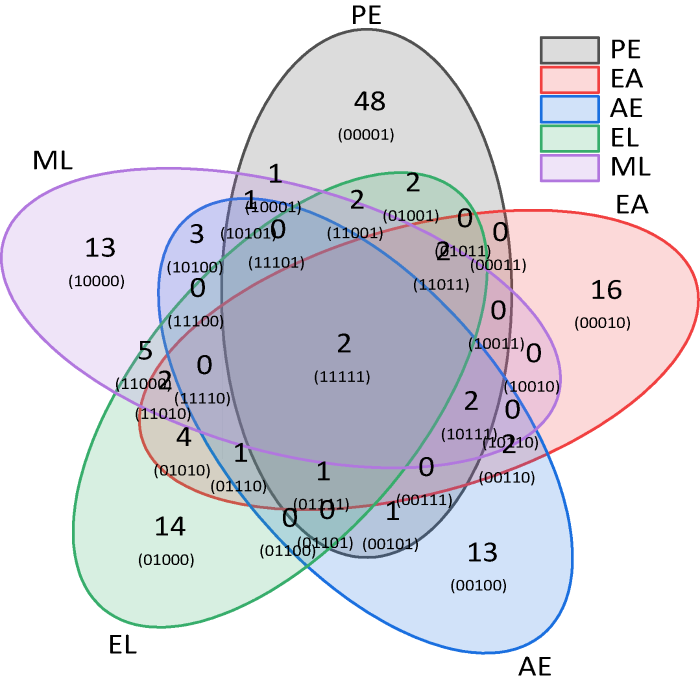
萃取物间共有化合物的数量可反映萃取剂对样品中化合物的分离效果,共有化合物数量越少,萃取分离就越充分。从5种萃取物化合物的韦恩图(图7)可知,分别独立存在于5种萃取物中化合物共104种,而2种或2种以上萃取物中共有的化合物为31种,说明5种萃取剂对样品中化合物的族组分分离效果较好。从萃取剂的极性差异来看,溶剂间极性相差越大,其萃取物中共有化合物就越少,而溶剂极性越相似,其萃取物中共有化合物就越多。如随着溶剂甲醇与乙醇、丙酮、乙酸乙酯和石油醚4种溶剂之间的极性差距增大,萃取物间共有的化合物分别为5种、3种、0种和1种(图5),总体上有减小的趋势;同样,除乙醇与丙酮外,乙醇与乙酸乙酯、石油醚的萃取物间共有化合物分别为4种和2种;丙酮与乙酸乙酯和石油醚萃取物间共有化合物分别为2种和1种,均表现出减小的趋势。
图7
图7
灵芝孢子粉5种萃取物中化合物的韦恩图
Fig. 7
Venn diagram of compounds in the extracts from Ganoderma lingzhi spore powder using petroleum ether (PE), ethyl acetate (EA), acetone (AE), ethanol (EL) and methanol (ML) as solvents.
实验结果显示灵芝孢子粉中酸类物质以不饱和脂肪酸为主,是人体中重要的脂肪酸,具有多种重要的生理作用。如在石油醚萃取物中含量最高的油酸是构成磷脂、甘油三酯的主要脂肪酸,还可以对黄曲霉毒素诱导的肝细胞损伤起保护作用(师文文等 2019);在甲醇萃取物中含量较高的亚油酸是一种重要的必需脂肪酸,人体合成很少甚至不能合成,必须从食物中摄取,动物实验表明亚油酸对治疗高血脂、动脉硬化和佐剂性关节炎有一定的疗效(衣丹等 2011;赵敏等 2012)。此外,芥酸酰胺对小鼠有一定的抗焦虑作用(宋灵云等 2017);油酸酰胺对关节炎、动脉粥样硬化、癌症有一定的治疗作用,还可以防止血栓形成,缓解水肿等(Hopps et al. 2012);棕榈酸可以在分子水平上调控心血管疾病、神经退行性疾病、炎症、癌症多种疾病的产生和发展(Fatima et al. 2019);棕榈酸甲酯可以通过诱导PPAR-α来预防非酒精性脂肪性肝炎(Zhang et al. 2019)。
灵芝三萜类是灵芝的主要活性成分,其中灵芝酸尤为重要(孙培龙等 2018),具有广泛的药理作用。灵芝酸A能够减弱小鼠中脂多糖诱导的肺损伤(Wan et al. 2019);灵芝酸F对人宫颈癌的细胞增殖有抑制作用(Liu & Zhong 2011);灵芝酸A、B、G、H均具有镇痛作用(Koyama et al. 1997)。灵芝中生物碱一般含量较低,但其具有抗炎、降血压、降低胆固醇等多种生理活性(姜芳燕等 2014),可有效提高小鼠免疫力( 程丽佳等2017 )。实验仅在乙醇、甲醇和水中鉴定出吡唑类、吡咯类及异喹啉类3种生物碱,这可能与生物碱类化合物含有极性较大的羟基或仲胺基团,易于与水、甲醇、乙醇形成氢键有关。在鉴定出的9种有机酸类化合物中,除石油醚萃取物中的前列腺素F0α外,其余8种均为不饱和脂肪酸。前列腺素F0α,又名二氢前列腺素F1α,是由前列腺素F1α中的双键加氢后形成的,其结构中含有多个羟基,仅被极性小的石油醚提取出来,说明前列腺素F0α极易溶于有机溶剂,这对于后续前列腺素类化合物的分离具有一定的参考意义。目前对前列腺素F1α、前列腺素F2α的药理作用研究相对较多,而对前列腺素F0α的相关研究较少。
本研究尝试采用不同极性溶剂的分级萃取方法以及利用气-质和液-质测定技术相结合对灵芝孢子粉的萃取物中化学成分进行分析与鉴定,鉴定出的化合物数量有较大增加。同时,利用GC/MS技术在灵芝孢子粉的萃取物中也鉴定出了一些具有药理作用的小分子化合物,因此灵芝中小分子化合物活性和功能也值得关注。
参考文献
Secondary metabolites from Ganoderma
DOI:10.1016/j.phytochem.2015.03.010 URL [本文引用: 1]
Species clarification of the prize medicinal Ganoderma mushroom “Lingzhi”
DOI:10.1007/s13225-012-0178-5
URL
[本文引用: 1]

"Lingzhi" is a mushroom that has been renowned in China for more than 2,000 years because of its claimed medicinal properties plus its symbolic fortune. "Lingzhi" has high economic value mostly as a dietary supplement in the modern market especially in East Asia, and its medicinal functions have become a hot study topic. For over a century, the highly prized medicinal fungus, known as "Lingzhi" in East Asia, has been assigned to Ganoderma lucidum, a species originally described from Europe. Molecular studies in recent years have revealed that the commercially cultivated 'G. lucidum' ("Lingzhi") in East Asia is a different species from the true G. lucidum. The present study aims to clarify the species identity of "Lingzhi" based on morphological studies and analysis of rDNA nuc-ITS sequences, and additional gene fragments of mt-SSU, RPB1, RPB2, and TEF1-alpha of "Lingzhi" were provided. All Ganoderma species that mostly resemble "Lingzhi" in phylogeny and /or morphology were included for analysis. We propose a new species G. lingzhi for "Lingzhi", which has an East Asia distribution. The most striking characteristics which differentiate G. lingzhi from G. lucidum are the presence of melanoid bands in the context, a yellow pore surface and thick dissepiments (80-120 mu m) at maturity. G. curtisii is most closely related to G. lingzhi in phylogeny and is from North America. Ganoderma flexipes, G. multipileum, G. sichuanense, G. tropicum and 'G. tsugae', are also closely related with G. lingzhi and are reported from China. These species are compared and discussed. 'Ganoderma tsuage' reported from China is determined as conspecific with G. lucidum, hence the distribution of G. lucidum extends from Europe to northeastern China.
Ganoderma spore powder in the role of tumor treatment
Research progress on wall-broken methods of Ganoderma lucidum spores and its pharmacological effects
Notes on the nomenclature of the most widely cultivated Ganoderma species in China
“Lingzhi” is one of the most important medicinal fungi, and it has been renowned and utilized in China for more than 2,000 years. Ganoderma lucidum was originally described for Britain specimens by William Curtis as Boletus lucidus in 1871. Patouillard first reported Ganoderma lucidum from China in 1907. Thereafter this scientific name (binomial name) has been used for the Chinese medicinal “Lingzhi” for more than 100 years. However, the recent taxonomical studies indicated the Chinese “Lingzhi” is different from G. lucidum in both phylogeny and morphology. The Chinese “Lingzhi” is an independent species, and its valid scientific name is G. lingzhi rather than G. lucidum. Ganoderma lingzhi has a wide distribution in warm temperate and subtropical East Asia, and it differs from G. lucidum by its pale yellow to sulphur yellow pore surface when fresh, the presence of melanoid bands in the context and thick dissepiments (80–120μm) at maturity, while G. lucidum has a distribution mostly in Europe, but also in northeast, northern, central and highland of southwest China, and it lacks melanoid bands in the context, and has a white to cream pore surface and thin dissepiments (40–80μm). Ganoderma sichuanense was originally described from Panzhihua of Sichuan Province, China. Based on the ITS sequence of its holotype, it differs from G. lingzhi in phylogeny. In addition, it was also recently found in Guangdong Province, China.
Palmitic acid is an intracellular signaling molecule involved in disease development
DOI:10.1007/s00018-019-03092-7 URL [本文引用: 1]
Fractional extraction and identification of the chemical components from Cordyceps cicadae product
Analysis of volatile components and alkaloids from sporoderm-broken spores of Ganoderma lucidum
Fruiting body, spores and in vitro produced mycelium of Ganoderma lucidum from Northeast Portugal: a comparative study of the antioxidant potential of phenolic and polysaccharidic extracts
DOI:10.1016/j.foodres.2011.12.009
URL
[本文引用: 1]

Ganoderma lucidum is one of the most extensively studied mushrooms due to its medicinal properties. Herein, a systematic study was carried out in order to compare the antioxidant activity of phenolic and polysaccharidic extracts from fruiting body, spores and mycelium, obtained in three different culture media, of G. lucidum from Northeast Portugal. Phenolic extracts were characterized using high-performance liquid chromatography coupled to photodiode array detection, while polysaccharidic extracts were hydrolysed and further characterized using HPLC and refraction index detection. In general, the phenolic extracts (Ph) proved to have higher antioxidant potential than their corresponding polysaccharidic extracts (Ps). Amongst phenolic extracts, FB-Ph provided the highest antioxidant activity (EC50 <= 0.6 mg/ml) and the highest content in total phenolics (similar to 29 mg GAE/g extract) and phenolic acids (p-hydroxybenzoic and p-coumaric acids). S-Ps was the polysaccharidic extract with the best antioxidant activity (EC50 <= 2 mg/ml); nevertheless, the highest levels of total phenolics were obtained in FB-PS (similar to 56 mg GAE/g extract), while the highest levels of total polysaccharides (similar to 14 mg PE/g extract) and individual sugars were observed in mycelia obtained from solid culture media, M-PDA-Ps and M-sMMN-Ps. The free radical scavenging properties, reducing power and lipid peroxidation inhibition of G. lucidum seemed to be correlated with phenolic compounds mostly in a free form, but also linked to polysaccharides. (C) 2011 Elsevier Ltd.
Enhanced vasorelaxant effects of the endocannabinoid-like mediator, oleamide, in hypertension
DOI:10.1016/j.ejphar.2012.03.027
URL
[本文引用: 1]

Oleamide is an endocannabinoid-like, fatty acid amide with structural similarities to anandamide. The cardiovascular effects of anandamide are enhanced in hypertension and we have now examined how hypertension affects responses to oleamide. Vasorelaxant responses to oleamide were significantly (P<0.001) enhanced in aortic rings from spontaneously hypertensive rats (SHRs), such that the maximal relaxation to oleamide was 40.3 +/- 3.5%, compared to 15.7 +/- 3.9% in normotensive Wistar Kyoto (WKY) controls. The augmented responses to oleamide in SHR arteries were unaffected by either inhibition of nitric oxide synthase (300 mu M L-NAME) or fatty acid amide hydrolase (1 mu M URB597) and independent of cannabinoid CB1 receptors or the endothelium. The enhanced responses to oleamide were opposed by pre-treatment with capsaicin (such that R-max was reduced to 9.8 +/- 1.5%) and this occurred independently of TRPV1 receptor and sensory nerve activity, as the TRPV1 antagonist capsazepine (1-5 mu M) and the cation channel inhibitor ruthenium red (10 mu M) had no effect on the responses to oleamide. However, inhibition of cyclooxygenase (10 mu M indomethacin) enhanced the responses in the WKY aortae, such that the responses were comparable to those in the SHR. The results suggest that the cyclooxygenase pathway has a role in modulating vasorelaxation caused by oleamide in normotensive aortae and that this is lost in hypertension, possibly as an adaptation to the increase in blood pressure. (C) 2012 Elsevier B.V.
LingzhiBaozifen (LZBF) attenuates stomach inflammation induced by ethanol in mice
Research prograss on active ingredients of Ganoderma sp
Biosynthesis of plant isoprenoids: perspectives for microbial engineering
DOI:10.1146/annurev.arplant.043008.091955 URL [本文引用: 1]
Antinociceptive components of Ganoderma lucidum
DOI:10.1055/s-2006-957658 URL [本文引用: 1]
Identification/quantification of multiple pesticide residues in food plants by ultra-high-performance liquid chromatography-time-of-flight mass spectrometry
DOI:10.1016/j.chroma.2009.11.098 URL [本文引用: 1]
Application and research progress of Ganoderma lucidum in respiratory diseases
Ganoderic acid Mf and S induce mitochondria mediated apoptosis in human cervical carcinoma HeLa cells
DOI:10.1016/j.phymed.2010.08.019
URL
[本文引用: 1]

In this work, the effects of a pair of positional isomer of ganoderic acids (GAS), namely ganoderic acid Mf (GA-Mf) and ganoderic acid S (GA-S) purified from the fermented mycelia of Ganoderma lucidum, on induction of cell apoptosis and the apoptotic pathway in HeLa cells were investigated. The results demonstrate that both isomers decreased cell population growth on various human carcinoma cell lines by MTT assay, while GA-Mf had better selectivity between normal and cancer cells. The flow cytometry analysis indicated that treatment of HeLa cells with GA-S caused cell cycle arrest in the S phase, while GA-Mf caused cell cycle arrest in the G1 phase. Compared with GA-S. GA-Mf had more potent increase in the number of early and late apoptotic cells. Treatment of HeLa cells with each isomer decreased the mitochondria membrane potential and caused the release of cytochrome c from mitochondria into the cytosol. In addition, stimulation of caspase-3 and caspase-9 activity was observed. The Bax/Bcl-2 ratio was also increased in GA-treated HeLa cells. The results demonstrated that both isomers GA-Mf and GAS induced apoptosis of human HeLa cells through a mitochondria mediated pathway, but they had the different cell cycle arrest specificity. The findings will be helpful to the development of useful cancer chemopreventive compounds from G. lucidum. (C) 2010 Elsevier GmbH.
Research on alkaloid metabolites from endophytic fungus Penicillium sp
Lanostane-type triterpenes from the sporoderm-broken spores of Ganoderma lucidum
DOI:10.1038/ja.2011.135 URL [本文引用: 1]
Ganoderma lucidum: a potent pharmacological macrofungus
DOI:10.2174/138920109789978757 URL [本文引用: 1]
Protective effect of oleic acid on aflatoxin- induced hepatocyte injury
The anxiolytic like effects of erucamide on mice
Analysis of triterpenoids in Ganoderma spore powder by UPLC-Q-TOF-MS
Ganoderma lucidum: an emerging medicinal model fungus for study of the biosynthesis of natural medicines
Research progress of triterpenes in Ganoderma lucidum
Current progress in the study on biosynthesis and regulation of Ganoderma triterpenoids
Pharmacological effects of organic acids in Chinese herbs and its application in cardiovascular diseases
Ganoderic acid A attenuates lipopolysaccharide-induced lung injury in mice
Research on effects of Ganoderma spore oil on angiogenesis
An analysis on nucleosides of spore powder produced by Ganoderma lingzhi
Antitumor and immunomodulatory effects of polysaccharides from broken-spore of Ganoderma lucidum
Progress of heterologous biosynthesis of terpenoids in engineered Corynebacterium glutamicum
Study on in vitro anti-tumor activity of triterpenoids from Ganoderma lucidum
Effect of conjugated linoleic acid in lowering blood lipids and anti-atherosclerosis
Development of a sulfonic gemini zwitterionic viscoelastic surfactant with high salt tolerance for seawater-based clean fracturing fluid
DOI:10.1016/j.ces.2019.06.061 URL [本文引用: 1]
The therapeutical effect of linoleic acid and methyl linoleate on adjuvant arthritis of rats
Neuroprotective effect of preadministration with Ganoderma lucidum spore on rat hippocampus
DOI:10.1016/j.etp.2010.12.011 URL [本文引用: 1]
Research progress on polysaccharide from Ganoderma lucidum spore powder
Application of GC-MS in traditional Chinese medicine research
中国灵芝学名之管见
灵芝是最重要的药用真菌之一,在我国已有2,000多年的记载和利用历史。虽然早在一百多年前法国真菌学家Patouillard就有给中国的灵芝冠上Ganoderma lucidum这一学名,并沿用至今,但随着分子生物学技术的不断发展,人们认识到过去外国人的定名并不正确。实际上,G. lucidum 是1871年由William Curtis根据采自英国的标本描述的新物种。最近的研究表明,我国广泛分布和栽培的灵芝与产于欧洲的G. lucidum不同,是一个独立的种,其合法的拉丁学名应为G. lingzhi。鉴于“灵芝”这一名称在中国已使用2,000余年,故建议“G. lingzhi”的汉语学名为“灵芝”(俗称赤芝),而灵芝属的模式种G. lucidum的汉语学名改为“亮盖灵芝”(俗称白肉灵芝或白灵芝)。灵芝Ganoderma lingzhi广泛分布于东亚暖温带和亚热带,其主要形态特征是孔口表面新鲜时浅黄色至硫磺色、成熟时菌肉中有黑褐色区带、管口壁厚度为80–120μm。亮盖灵芝G. lucidum主要分布于欧洲和亚洲,在我国分布于东北、华北、华中和西南海拔较高地区,其孔口表面新鲜时白色至奶油色、成熟时菌肉中无黑褐色区带、管口壁厚度为40–80μm。四川灵芝G. sichuanense尽管其担孢子与灵芝G. lingzhi相似,但基于其模式标本ITS序列的系统发育研究表明,该种与灵芝不同,是个独立的种,且在广东也有发现。
内生真菌Penicillium sp.生物碱类次级代谢产物研究