黄曲霉是一类能够通过空气与土壤传播的植物和动物致病性丝状真菌,在12-48℃的温度下均可以生长,最佳生长温度为37℃,和人体的正常体温相同,因此增加了对人体致病的可能性(Diener et al. 2003)。黄曲霉作为仅次于烟曲霉的曲霉病病原菌,产生的次级代谢产物含有聚酮类化合物,是一类强致癌物质,会严重影响人类和动物健康(Hedayati et al. 2007)。黄曲霉能够在收获、运输、贮藏等过程中侵染玉米,花生和坚果等多种富含油脂的农作物种子,并产生大量次级代谢产物——黄曲霉毒素(aflatoxin)(Stevens et al. 2000;Bhatnagar et al. 2006;Hedayati 2007)。黄曲霉毒素属于黄曲霉次生代谢产物的一类,主要分为4种:B1、B2、G1和G2,字母表示它们在紫外线(蓝色或绿色)下的荧光颜色,数字表示它们在薄层色谱上的相对迁移距离,其中以AFB1毒性最强(Bhatnagar et al. 2006;Klich 2007)。黄曲霉能够通过其产生的无性孢子(分生孢子)进行传播。在逆境下,黄曲霉还能分化产生菌核,有研究表明菌核是紧密的菌丝体团,能够帮助其渡过不利环境,在适宜的环境中重新分化萌发(Diener et al. 2003)。
真核生物在细胞表面和细胞核之间通过特殊的信号转导途径来响应外界环境的变化。模式真菌酿酒酵母Saccharomyces cerevisiae中存在高度保守的由3个级联蛋白激酶组成的促分裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)途径。该途径中的MAPKinase kinase kinase(MAPKKK)使第二种激酶MAPKinase kinase(MAPKK)磷酸化,最终激活MAPK的磷酸化,磷酸化的MAPK能够进入细胞核激活相关核内靶蛋白基因的表达,应对外界环境变化,维持细胞稳态(Marshall 1994;Yu 2010)。在酿酒酵母中,高渗透性甘油促分裂原激酶信号转导途径(high osmolarity glycerol mitogen- activated protein kinase,HOG-MAPK)中高度保守的Rho型GTP酶Cdc42蛋白及鸟苷酸转换因子Cdc24蛋白与支架蛋白Bem1相互作用并形成三元复合物,共同发挥能量交换的作用。该复合物中包含的Cdc42蛋白的下游效应子PAK激酶(P21-activated kinase)Cla4,共同参与调节酵母形态发生(Bose et al. 2001)。PAK激酶在真核生物中是保守的,该激酶家族调节促分裂原活化蛋白激酶信号传导、细胞周期进程和细胞形态发生(Manser et al. 1994)。Cla4信号分子是p21活化蛋白激酶(PAK)家族的成员之一,Cla4能与Cdc42相互作用且受其调控(Marshall 1994;Keniry et al. 2004)。同时,Cla4的磷酸化能够激活Ste11的磷酸化进而通过HOG-MAPK级联将外界信号传递到细胞核内,引起核内应对渗透胁迫的相关基因转录表达(Keniry & Sprague 2003)。研究表明,玉米黑粉病Ustilago maydis中cla4基因敲除突变体菌丝以分支状的形式生长,并在隔膜处的收缩和裂变中分裂(Leveleki et al. 2004)。耶氏解脂酵母Yarrowia lipolytica cla4基因的敲除突变体虽然并不致死,但完全丧失了菌丝形成和侵染的能力,同时cla4基因的缺失导致了菌株细胞壁中几丁质的异常分布,表明在解脂耶氏酵母中Cla4激酶可能在维持细胞极性的过程中发挥作用(Szabo 2001)。玉米小斑病菌Bipolaris maydis中PAK样激酶Cla4是其生长、致病性及有性/无性发育的主要调节剂,另一种同源PAK样激酶Ste20起次要作用(Kitade et al. 2019)。本研究的主要目的是探究黄曲霉的cla4基因在形态发育、应激反应和致病性等方面的功能。黄曲霉cla4基因功能的研究有助于新技术开发以控制由黄曲霉侵染造成的直接损失,同时可针对性开发黄曲霉真菌毒素抑制剂,以增强宿主抵抗真菌侵害的能力。
1 材料与方法
1.1 菌株和培养基
表1 本研究使用到的菌株
Table 1
| 菌株 Strain | 基因型 Genotype | 参考文献 References |
|---|---|---|
| A. flavus CA14 PTS | Δku70, ΔniaD, ΔpyrG | Chang et al. 2010 |
| A. flavus wild-type (WT) | Δku70, ΔniaD, ΔpyrG::AfupyrG | This study |
| A. flavus ΔAflcla4 | Δku70, ΔniaD, ΔAflcla4::AfupyrG | This study |
表2 本研究使用的培养基
Table 2
| Media | Composition (Add 1.5% agar to solid media) (/L) |
|---|---|
| PDA | 39g difco potato dextrose agar (BD, USA) |
| YES | 1g MgSO4, 20g yeast extract, 150g sucrose |
| WKM | 30g Sucrose, 2g NaNO3, 0.5g KCl, 1g K2HPO4·3H2O, 0.5g MgSO4·7H2O, 0.01g FeSO4·7H2O (pH 5.5) |
| MM | 1.84g/L ammonium tartrate, 6g/L NaNO3, 0.52g/L KCl, 0.52g/L MgSO4·7H2O, 1.52g/L KH2PO4, 1mL trace element |
| GMM | 1.84g/L ammonium tartrate, 6g/L NaNO3, 0.52g/L KCl, 0.52g/L MgSO4·7H2O, 1.52g/L KH2PO4, 1mL trace element, 10g/L glucose |
| CM | 6g/L peptone, 6g/L yeast extract, 10g/L sucrose |
1.2 生物信息学分析
在NCBI的数据库(
1.3 黄曲霉cla4基因敲除菌株构建
采用同源重组的方法对黄曲霉Aflcla4基因进行敲除,将潮霉素抗性基因pyrG作为筛选标记基因。与目的基因的5’及3’端同源臂通过overlap连接,再把连接好的片段导入黄曲霉CA14 PTS原生质体内,通过PEG介导法实现基因敲除,由于出发菌株黄曲霉CA14 PTS不含pyrG基因不能在基本培养基上生长,转化板至于37℃培养箱培养2-3d后,挑取单一的转化子到新的平板上再培养2-3d,提取转化子基因组后进行AP片段(5’端引物:cla4-P2,3’端引物:pyrG-907-R)、BP片段(5’端引物:pyrG-919-F,3’端引物:P7)以及开放阅读框(open reading frame,ORF)(5’端引物:cla4-P9,3’端引物:P10)验证。且同时在cDNA水平上验证ORF(5’端引物:cla4-qRT-P9,3’端引物:cla4-qRT- P10),实验所用引物序列见表3。
表3 本研究过程中所用引物
Table 3
| Primer | Sequence (5’-3’) | Characteristics |
|---|---|---|
| cla4-P1 | AAAGTGGTTCAATGCCTAC | 5’UTR of Aflcla4 |
| cla4-P3 | GGGTGAAGAGCATTGTTTGAGGCGGGACAAAGCCATCAGTA | |
| pyrG-F | GCCTCAAACAATGCTCTTCACCC | A. fumigatus pyrG |
| pyrG-R | GTCTGAGAGGAGGCACTGATGC | |
| cla4-P6 | GCATCAGTGCCTCCTCTCAGACAACTGAGACGGGCTTTAC | 3’UTR of Aflcla4 |
| cla4-P8 | CTCGATGTGATCCACCTAC | |
| cla4-P2 | CTCGTCCAGGCTCGTTA | Fusion PCR |
| cla4-P7 | ATCTTCGTCCGCAGGTA | |
| cla4-P9 | ATTGCTCCTCCAAGACCG | ORF validates primers |
| cla4-P10 | GCTCCTTTCGTGGCTGAC | |
| cla4-qRT-P9 | TGCTCTCACAGATGTCATTG | |
| cla4-qRT-P10 | GTCCGTTGTTCTTCTTCCA | cla4 mutant screen |
| pyrG-907-R | ATGACGGCGATGTAGGGA | |
| pyrG-919-F | CGACATCCTCACCGATTTCA | RT-PCR for AFB1 biosynthesis |
| aflQ-qRT-F | GTCGCATATGCCCCGGTCGG | |
| aflQ-qRT-R | GGCAACCAGTCGGGTTCCGG | |
| aflR-qRT-F | AAAGCACCCTGTCTTCCCTAAC | RT-PCR for conidial biosynthesis |
| aflR-qRT-R | GAAGAGGTGGGTCAGTGTTTGTAG | |
| abaA-qRT-F | TCTTCGGTTGATGGATGATTTC | |
| abaA-qRT-R | CCGTTGGGAGGCTGGGT | |
| brlA-qRT-F | GCCTCCAGCGTCAACCTTC | RT-PCR for sclerotial biosynthesis |
| brlA-qRT-R | TCTCTTCAAATGCTCTTGCCTC | |
| nsdC-qRT-F | GCCAGACTTGCCAATCAC | |
| nsdC-qRT-R | CATCCACCTTGCCCTTTA | |
| nsdD-qRT-F | GGACTTGCGGGTCGTGCTA | |
| nsdD-qRT-R | AGAACGCTGGGTCTGGTGC | |
| actin-F | ACGGTGTCGTCACAAACTGG | |
| actin-R | GCGTATCGTCGTTACCTCATC |
1.4 黄曲霉菌落生长速率测定
挑取PCR验证正确的两株Aflcla4 敲除突变体和野生型(wild type,WT)菌株接种在PDA培养基上,在37℃黑暗培养5d后进行孢子洗脱收集。取1μL浓度为107个/mL分生孢子悬浮液分别接种于PDA、MM、GMM、CM培养基上,生长3d后测量菌落直径,洗脱后进行孢子量统计。每个菌株设置5个平行,实验重复3次。
1.5 黄曲霉分生孢子梗形态观察
取1μL浓度为107个/mL的野生型与Aflcla4敲除突变体分生孢子悬浮液接种于PDA培养基(5mL),37℃黑暗培养3d后,刮取培养基表面菌落,切取长条形培养基置于载玻片上。放入铺上滤纸的培养皿中,加入1mL无菌水37℃培养10h后置于荧光显微镜(Leica,Germany)下观察并拍照。每个菌株设置5个平行,实验重复3次。
1.6 外界环境胁迫对黄曲霉的影响及测定
取1μL浓度为107个/mL的野生型与Aflcla4敲除突变体分生孢子悬浮液接种于含有100μg/mL刚果红(congo red,CR)、不同浓度NaCl、不同水活度、以及10mmol/L H2O2的YES固体培养基上,37℃黑暗培养3d后观察并测量菌落直径。每个菌株设置5个平行,实验重复3次。
1.7 黄曲霉菌核产生能力的测定
取1μL浓度为107个/mL的野生型与Aflcla4敲除突变体分生孢子悬浮液接种于WKM培养基上,每个菌株5个重复;待孢子液自然风干后将培养皿封口,并倒置于37℃培养箱,黑暗条件下培养7d。用75%的酒精喷洗菌落表面的孢子和菌丝,喷洗前后均进行拍照,沿菌落直径打5个1cm2的孔,统计菌核数目。
1.8 黄曲霉毒素合成的测定
取30μL浓度为107个/mL的野生型与Aflcla4敲除突变体分生孢子悬浮液接种于10mL YES液体培养基,在29℃培养箱黑暗条件下培养7d,每个菌株设置3个重复。取0.7mL含有黄曲霉毒素的培养基至1.5mL EP管并加入等体积的二氯甲烷,涡旋混匀5min。静置分层后吸出上层培养基;待剩余有机层在通风橱风干后加入50μL二氯甲烷进行复溶。吸取5μL复溶后的溶液于硅胶板(青岛海洋化工)点样(以黄曲霉AFB1标品做对照),在层析液(氯仿:丙酮=9:1)中进行TLC薄层层析(Zhou et al. 2009);于化学发光成像系统(Gene公司)中曝光检测。每个菌株设置5个平行,实验重复3次。
1.9 黄曲霉致病性的测定
选取新鲜饱满的花生和玉米种子放入锥形瓶中,加入75%乙醇(0.2%的曲拉通溶液配制)浸泡后于180r/min摇床洗涤5min,再用曲拉通溶液冲洗3次。每个培养皿中放置两张无菌滤纸,加入曲拉通溶液保持湿润,每个培养皿中平放7粒种子。取1μL浓度为107个/mL野生型与Aflcla4敲除突变体分生孢子悬浮液接种于种子表面,待菌液风干后放置于29℃黑暗培养箱培养7d。每个菌株设置5个平行,实验重复3次。培养过程中,注意观察滤纸的湿度,适当加水。收集侵染后的种子,经无菌水洗脱后,取等体积洗脱液统计孢子数量。通过上述1.8部分相同方法提取被侵染种子中的毒素。
1.10 细胞隔膜检测
取1μL浓度为107个/mL野生型与Aflcla4敲除突变体分生孢子悬浮液接种于1.5mL EP管中(提前加入550μL YES液体培养基),37℃ 180r/min培养24h,离心收集后使用PBS润洗3次。用150μg/mL荧光增白剂CFW(calcofluor white)冰上染色10min。离心收集后用PBS润洗2次,置于荧光显微镜(Leica,Germany)下观察并拍照。每个菌株5个重复,实验重复3次。
1.11 RNA提取与荧光定量PCR(qRT-PCR)
取1μL浓度为107个/mL的野生型与Aflcla4敲除突变体分生孢子悬浮液接种于铺有玻璃纸的PDA、YES、WKM固体培养基上。37℃培养箱中黑暗培养12h,液氮研磨菌丝直至粉末。使用RNA试剂盒(天漠,中国)分别提取RNA,随后通过反转录试剂盒(Trans,中国)反转录成cDNA。使用PikoReal 96荧光定量PCR仪(Thermo scientifics,USA)检测相关基因相对表达量。
2 结果与分析
2.1 Aflcla4的生物信息学分析
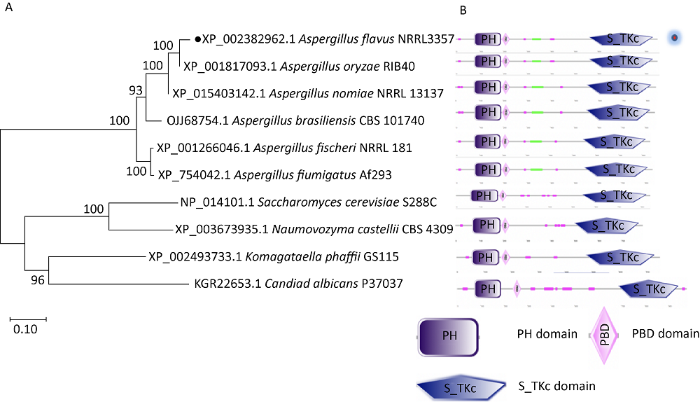
将酿酒酵母中Cla4蛋白的氨基酸序列(Saccharomyces cerevisiae S288C,NP_014101.1),在黄曲霉NRRL3357全基因组数据库中进行BLAST,比对获得黄曲霉(Aspergillus flavus NRRL3357,XP_002382962.1)Cla4蛋白激酶。以黄曲霉Cla4氨基酸序列进行同源蛋白比对,获得烟曲霉(Aspergillus fumigatus Af293,XP_754042.1)、米曲霉(Aspergillus oryzae RIB40,XP_001817093.1)、根瘤菌(Komagataella phaffii GS115,XP_002493733.1)、纳莫付酵母(Naumovozyma castellii CBS 4309,XP_003673935.1)和白色念珠菌(Candida albicans P37037,KGR22653.1)等Cla4蛋白的氨基酸序列。采用MEGA7.0软件对上述不同物种的Cla4蛋白构建进化树并分析其亲缘关系。分析结果表明,曲霉属中Cla4蛋白具有高度保守性,且A. flavus 与A. oryzae的亲缘关系最近(图1A)。通过在线网站(http:// smart.embl-heidelberg.de/)进行结构域分析发现,被检测菌株的Cla4蛋白中均含有3个保守的结构域:PH domain(Pleckstrin homology domain)、PBD domain(P21-Rho-binding domain)和S_TKc domain(serine/threonine protein kinases,catalytic domain)(图1B)。
图1
图1
Cla4蛋白的生物信息学分析 A:不同物种中Cla4同源蛋白的系统进化分析;B:不同物种中Cla4蛋白的功能结构域分析
Fig. 1
Bioinformatic analysis of Cla4 protein from different fungi. A: Evolutionary relationship analysis of Cla4 homologous proteins in different species; B: Domain analysis of Cla4 proteins in different species.
2.2 Aflcla4敲除突变体的构建
图2
图2
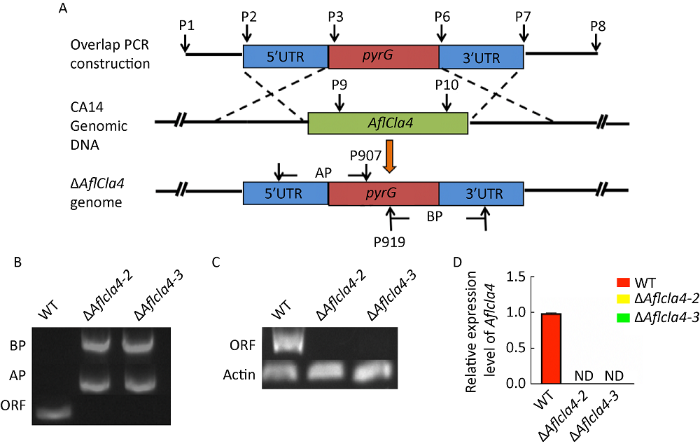
黄曲霉Aflcla4基因缺失菌株的构建 A:Aflcla4基因敲除原理示意图;B:PCR扩增ΔAflcla4的ORF,AP和BP片段(引物为P9/P10,P2/P907和P919/P7);C:Aflcla4 敲除菌株的反转录PCR验证,actin基因为对照;D:ΔAflcla4和WT菌株的qRT-PCR表达量分析,柱状图代表Aflcla4基因在菌株中的相对表达量. 2-ΔΔCt法用于计算目标基因的表达量,β-actin基因作为内参. ND:未检测到
Fig. 2
Generation of cla4 deletion mutant in Aspergillus flavus. A: Schematic illustration for Aflcla4 disruption; B: The results of the PCR analysis of ΔAflcla4, ORF (open reading frame) AP and BP fragments were amplified with a couple of primers P9/P10, P2/P907 and P919/P7 respectively. C: Verification of Aflcla4 deletion strain with reverse transcription-PCR. Actin gene was used as an endogenous control. D: qRT-PCR analysis of the expression level of Aflcla4 gene in ΔAflcla4 mutant and WT (wild-type) strains. The histogram (D) indicates relative expression level of Aflcla4 gene in strains. The 2-ΔΔCt method was used to evaluate target gene expression levels, which were relative to the expression level of the β-actin reference gene. ND: Not detected.
2.3 Aflcla4参与黄曲霉营养生长与孢子生成的调控
图3
图3
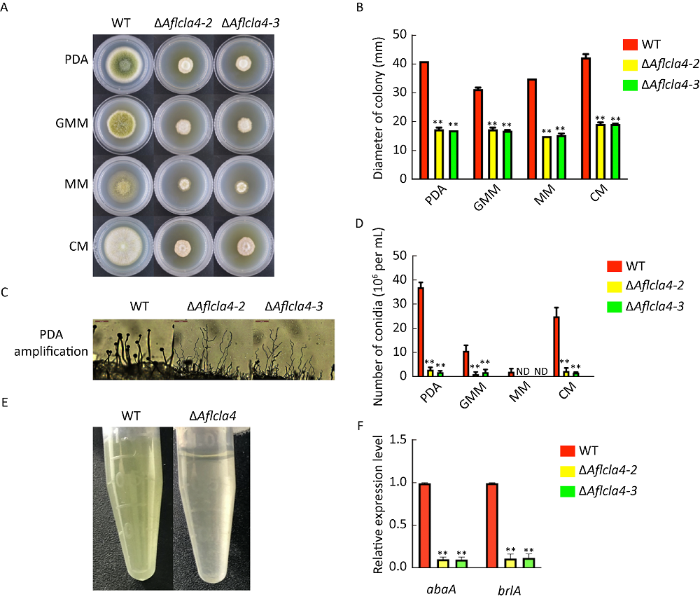
黄曲霉Aflcla4缺失株的菌落生长直径及分生孢子相关表型分析 A:37℃条件下WT、ΔAflcla4-2 和ΔAflcla4-3菌株在PDA、GMM、MM和CM培养基培养3d后的菌落形态;B:菌落直径统计;C:分生孢子梗显微观察(200×);D:不同培养基上,WT和敲除菌株的分生孢子生产量统计;E:WT和敲除菌株的孢子悬浮液;F:abaA和brlA基因的相对表达量. 星号代表WT和其他菌株之间有显著性差异,**P≤0.01
Fig. 3
Roles of Aflcla4 deleted strains in vegetative growth and conidial formation. A: Colony morphology of the wild-type, the single deletion mutants ΔAflcla4-2 and ΔAflcla4-3 on PDA, GMM, MM and CM media at 37°C for 3d; B: Colony diameters of strains; C: Microscopic analysis of conidial structures (200×); D: Indicates the number of conidia produced by WT and deletion strains; E: Different colors of WT and mutant strains’ conidial suspension (108/mL); F: Relative expression level of abaA and brlA genes in strains. Asterisks indicate significant difference between WT and other strains, ** P≤0.01.
2.4 Aflcla4参与调控黄曲霉渗透压胁迫
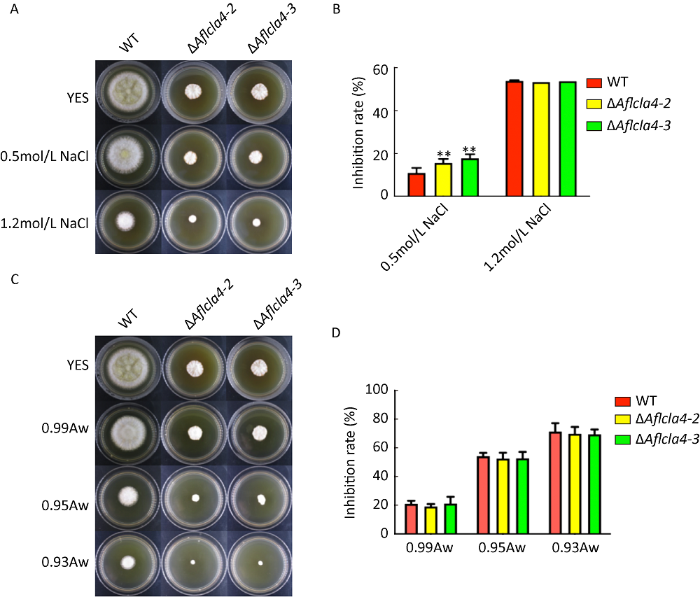
在酿酒酵母中,Cla4参与高渗透压HOG-MAPK通路(Tatebayashi et al. 2006),因此我们也探究了渗透压对黄曲霉Aflcla4敲除株的影响。通过将WT与ΔAflcla4新鲜孢子液分别接种于添加了0.5mol/L与1.2mol/L NaCl的YES固体培养基上,在37℃黑暗培养箱中培养3d后测量菌落直径,比较不同菌株在渗透压胁迫下的生长情况。结果表明,在低渗透压0.5mol/L NaCl条件下,ΔAflcla4相比野生型生长抑制率显著上升(P≤0.01),但在1.2mol/L高渗透压胁迫下,两者生长抑制率没有显著差别(图4A,4B)。且在0.99 Aw、0.95 Aw、0.93 Aw水活度条件下生长抑制率没有显著差异(图4C,4D)。说明Aflcla4在0.5M Na+盐离子胁迫条件下参与了黄曲霉渗透压调节过程,但是在1.2mol/L Na+盐离子与各测试水活度胁迫下,并没有参与渗透压调节过程。
图4
图4
黄曲霉Aflcla4缺失株在渗透压胁迫下的菌落表型 A和C:WT、ΔAflcla4-2和ΔAflcla4-3在NaCl和Aw渗透胁迫处理下的表型;B和D:渗透胁迫下的生长抑制率统计,**P≤0.01
Fig. 4
Colony phenotype and growth inhibition rate of Aflcla4-deleted strains of Aspergillus flavus under osmotic stress. A and C: The colony morphology of wild type (WT), ΔAflcla4-2 and ΔAflcla4-3 strains under osmotic stress induced by NaCl (sodium chloride) and Aw (0.99, 0.95 and 0.93); B and D: The mycelial growth inhibition rate of the strains under osmotic stress, ** P≤0.01.
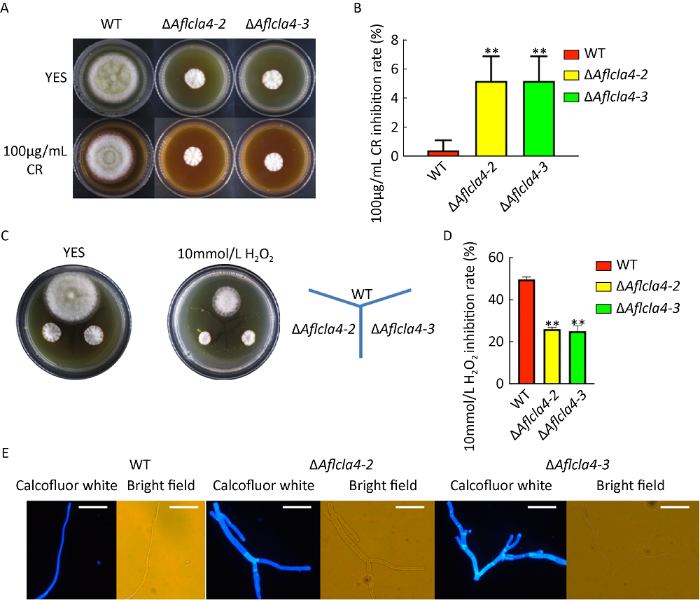
2.5 Aflcla4影响黄曲霉细胞壁完整性和活性氧产生
据报道,丝状真菌烟曲霉通过GTP酶Cdc42将Cla4蛋白招募到细胞膜上发挥作用,且对活性氧产生影响(Leberer et al. 1997)。为了探究在黄曲霉中cla4基因是否存在类似的功能,将野生型与ΔAflcla4新鲜孢子液接种于含有100μg/mL CR和10mmol/L H2O2的YES固体培养基上,37℃黑暗培养3d后测量菌落直径。结果表明,在添加了CR的YES培养基上,ΔAflcla4和野生型生长均受到抑制(图5A,5C)。抑制率统计结果表明,敲除株的抑制率显著高于野生型(P≤0.01)。但是在H2O2刺激下,ΔAflcla4生长抑制率相比野生型显著降低(图5B,5D),表明该基因的缺失使黄曲霉更加耐受氧化胁迫。采用CFW对细胞几丁质进行染色后发现,ΔAflcla4菌株相比野生型隔膜数量增加,几丁质呈现无规则聚集现象且菌丝极化生长(图5E)。以上结果说明Aflcla4与细胞壁完整性通路具有相关性。
图5
图5
ΔAflcla4应对细胞壁胁迫及氧化胁迫的情况 A和C:WT、ΔAflcla4-2和ΔAflcla4-3在细胞壁胁迫(100μg/mL CR)和氧化胁迫(100μg/mL CR)下的生长表型;B和D:细胞壁胁迫和氧化胁迫条件下WT、ΔAflcla4-2和ΔAflcla4-3菌株的生长抑制率;E:菌丝隔膜的显微观察(400×),标尺=1mm,**P≤0.01
Fig. 5
Effects of cell wall stress and oxidative stress on wild-type (WT) and mutants (ΔAflcla4-2 and ΔAflcla4-3) of Aspergillus flavus. A and C: The morphology of WT, ΔAflcla4-2 and ΔAflcla4-3 strains under cell wall stress (100μg/mL CR) and oxidative stress (10mmol/L H2O2); B and D: The mycelial growth inhibition of WT, ΔAflcla4-2 and ΔAflcla4-2-3 strains under cell wall stress and oxidative stress; E: Microscopic examination of the hyphal septum of each strain (400×). Scale bars=1mm,** P≤0.01.
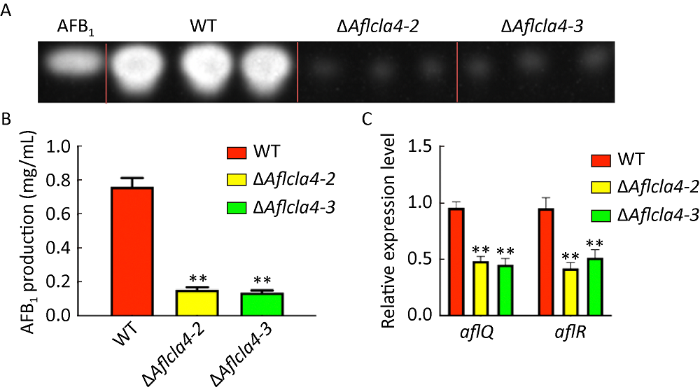
2.6 Aflcla4参与调控黄曲霉毒素合成
黄曲霉因能够产生危害人类健康的黄曲霉毒素而备受人们重视,其中以AFB1危害最大(Diener et al. 2003)。将野生型与ΔAflcla4菌株分生孢子分别接种于液体YES培养基中,放置于29℃黑暗培养7d后,使用二氯甲烷抽提黄曲霉毒素,通过TLC法检测不同菌株AFB1的产生情况。结果表明,ΔAflcla4相比野生型AFB1毒素合成量显著降低(P≤0.01)(图6A,6B)。通过qRT-PCR对黄曲霉产毒簇中结构基因(aflQ)和产毒调控基因(aflR)表达量进行测定,结果表明,敲除株中aflQ及aflR表达量与毒素合成量一致,均显著降低(图6C)。以上结果说明Aflcla4正调控黄曲霉毒素合成。
图6
图6
ΔAflcla4对黄曲霉毒素合成的影响 A:TLC薄层层析检测黄曲霉毒素生产量;B:AFB1产量统计;C:产毒相关基因相对表达量. 星号代表WT和其他菌株之间有显著性差异,**P≤0.01
Fig. 6
Roles of Aflcla4 on aflatoxin biosynthesis. A: Thin-layer chromatography analysis of aflatoxin production in the indicated strains; B: Analysis of aflatoxin B1 (AFB1) production; C: Relative expression levels of two aflatoxin biosynthesis-related genes. Asterisks indicate significant differences between the wild-type and other strains, **P≤0.01.
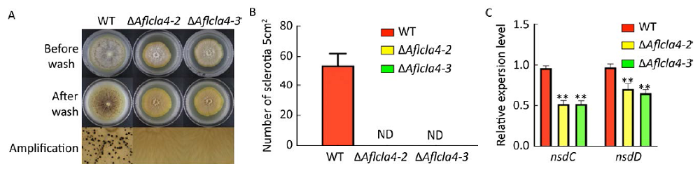
2.7 Aflcla4参与调控黄曲霉菌核生成
图7
图7
Aflcla4对黄曲霉菌核形成的影响 A:WKM培养基上菌核形成情况,下部显示平板局部放大图像;B:菌株菌核形成数量统计;C:菌核形成相关基因(nsdC和nsdD)相对表达量统计,所有菌株37℃条件下培养在WKM培养基平板,7d后用75%酒精喷洗平板. 星号代表WT和其他菌株之间有显著性差异,**P≤0.01. ND:未检出
Fig. 7
Effects of Aflcla4 deletion on sclerotial formation. A: Colony morphology of the strains producing sclerotia; magnified image of each plate is showed on the bottom; B: Analysis of sclerotia produced by the wild-type and two mutant strains; C: Relative expression levels of two genes related to sclerotial formation (nsdC and nsdD). All strains were cultured on the WKM medium at 37°C. After incubation for seven days, cultures were washed with 75% ethanol. Asterisks indicate significant differences between the wild-type and other strains (**P≤0.01). ND: No detected.
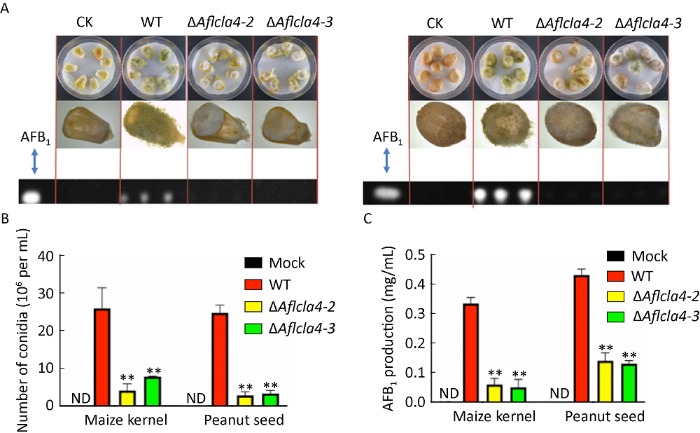
2.8 Aflcla4影响黄曲霉致病性
花生和玉米是我国重要的经济作物,黄曲霉能够在收获、运输、贮藏等过程中侵染花生和玉米,造成巨大的经济损失(Amaike & Keller 2011)。因此,研究Aflcla4基因对它们的致病能力尤为重要。将野生型与ΔAflcla4分别接种于经过处理的花生与玉米种子表面,置于29℃黑暗条件下培养7d后,统计侵染后产生的孢子数量和AFB1合成量。结果显示,相比野生型菌株,ΔAflcla4对花生和玉米的致病性下降,花生、玉米表面菌丝呈白色稀疏状,且不能完全覆盖花生与玉米表面(图8A),产孢量也显著降低(P≤0.01)(图8B)。同样,侵染花生、玉米后黄曲霉毒素含量相比野生型也成倍降低(图8C)。证明Aflcla4是黄曲霉保持完整侵染能力所必须的。
图8
图8
ΔAflcla4突变体对花生和玉米的侵染 A:WT和Aflcla4敲除菌株在29℃黑暗条件下侵染花生、玉米的表型分析;B:花生、玉米表面孢子量统计;C:侵染后花生、玉米黄曲霉毒素产生量统计. 星号代表WT和其他菌株之间有显著性差异,**P≤0.01
Fig. 8
Effects of Aflcla4 on pathogenicity of Aspergillus flavus to peanuts and maize. A: Phenotype analysis of WT and gene deletion strains cultured on peanuts and maize at 29°C in dark for seven days; B: Quantitative analysis of conidia collected from infected crop seeds; C: Thin-layer chromatography analysis of aflatoxin production in infected crop seeds. Asterisks indicate significant differences between the wild-type and other strains (**P≤0.01).
3 讨论
本研究发现Aflcla4基因在黄曲霉生长、形态发生和致病性等方面都起到了重要作用。黄曲霉ΔAflcla4敲除突变体与WT相比,在生长阶段菌丝呈极化无规则分支生长,这与在玉米黑粉病中观察到的突变体菌丝在隔膜处收缩裂变结果相似(Keniry & Sprague 2003)。在解脂耶氏酵母菌中研究发现,cla4基因的敲除突变体菌株几丁质在细胞质内异常聚集分布(Leveleki et al. 2004),本研究同时也发现用CFW染色后,ΔAflcla4与野生型相比菌丝隔膜数量明显增加,且观察到几丁质的异常无规则聚集现象发生,这可能是导致其极性不规则分裂的原因之一。在繁殖方面,ΔAflcla4的敲除突变体几乎完全丧失产孢能力,这与玉米小斑病菌中cla4基因能够控制菌株有性/无性生殖状态,从而影响子囊孢子生成的结果一致(Szabo 2001)。除了对分生孢子的影响外,本研究还确定了黄曲霉中cla4基因对菌核形成的影响。在酿酒酵母中已经证明Cla4激酶与下游Ste11之间形成蛋白复合体,传递磷酸化信号(Tatebayashi et al. 2006;Ren et al. 2016),而在黄曲霉中ste11基因敲除突变体与cla4基因敲除突变体一样不能产生菌核(Tatebayashi et al. 2006;Ren et al. 2016),这似乎暗示着这两个基因在调控菌核形成过程的潜在作用。
cla4基因在黄曲霉和其他丝状真菌中的重要功能是对渗透应激的响应。在酿酒酵母中cla4和另一个GTP酶Cdc42效应子ste20基因能够共同调节磷酸戊糖途径和糖酵解甘油合成途径中关键酶Gpd1蛋白的表达。敲除突变体Δcla4在低渗透压胁迫下,其胞内与控制甘油合成相关的Gpd1基因的表达降低(Joshua & Hofken 2019)。有趣的是,本研究也发现在低渗透胁迫条件下ΔAflcla4的生长抑制率明显高于野生型,这可能与AflCla4在细胞内应对渗透胁迫可正调控甘油合成存在联系。同时研究发现,在高浓度氧化胁迫刺激后,ΔAflcla4生长抑制率低于野生型,对氧化胁迫表现出的耐受性与黄萎病病原菌大丽轮枝菌Verticillium dahliae中Vdcla4参与调控ROS途径,调节其生长具有相似之处(Joshua & Hofken 2019)。在酿酒酵母中对Δcla4敲除突变体进行有丝分裂周期测定发现,大部分细胞分裂会停留在G1/S期,证明了其对细胞分裂周期的影响(Benton et al. 1997;Tian et al. 2015)。本研究发现,使用细胞壁损伤剂荧光白(CFW)染色后观察到细胞壁隔膜的增加和几丁质分布变化,且刚果红(CR)刺激后ΔAflcla4生长受到明显的抑制,猜测其在黄曲霉细胞壁正常合成功能上的损伤有可能是影响细胞分裂的原因之一,但是否直接参与了细胞壁完整性(CWI)通路,还需要进一步的研究确定。
病原真菌的致病性一直是备受关注的焦点,双态性白色念珠菌的致病性在Cacla4敲除突变体中,表现出对小鼠致病毒力的缺失(Leberer et al. 1997)。而在麦角菌Claviceps purpurea体外感染试验显示,Δcla4无法穿透植物表面,即使在7d后,也观察不到菌丝生成(Rolke & Tudzynski 2008)。相似的,本研究发现黄曲霉ΔAflcla4感染植物宿主的能力显著降低。同时,致病性方面的缺陷也表现在其次级代谢产物黄曲霉毒素AFB1产量的降低。ΔAflcla4菌株严重的生长缺陷,可能是导致其致病性下降的主要原因。总之,这些结果都表明cla4基因参与了病原真菌的致病过程,同时对致病性的影响巨大。
目前,国内外尚未见对病原菌黄曲霉菌中PAK样激酶Cla4的相关研究报道。本研究通过Aflcla4基因的缺失,探索了其对黄曲霉营养生长、生殖生长、毒素合成、耐受能力和致病性等方面的影响。发现了黄曲霉菌中AflCla4激酶可通过调节相关基因的表达进而影响细胞生长发育、次级代谢等生理过程,在黄曲霉中发挥了重要的生物学功能。
参考文献
Cla4p, a Saccharomyces cerevisiae Cdc42p-activated kinase involved in cytokinesis, is activated at mitosis
Understanding the genetics of regulation of aflatoxin production and Aspergillus flavus development
Assembly of scaffold-mediated complexes containing Cdc42p, the exchange factor Cdc24p, and the effector Cla4p required for cell cycle-regulated phosphorylation of Cdc24p
Development and refinement of a high-efficiency gene-targeting system for Aspergillus flavus
DOI:10.1016/j.mimet.2010.03.010
URL
PMID:20298723
[本文引用: 1]

An efficient gene-targeting system based on impairment of the nonhomologous end-joining pathway and the orotidine monophosphate decarboxylase gene (pyrG) in Aspergillus flavus was established. It was achieved by replacing the ku70 gene with the Aspergillus oryzae pyrithiamine resistance (ptr) gene and by inserting the Aspergillus parasiticus cypA gene into the pyrG locus. The utility of this system was demonstrated by disruption of nine candidate genes for conidial pigment biosynthesis. The gene-targeting frequencies ranged from 80 to 100%. Two linked genes on chromosome 4, wA and olgA, were confirmed to be involved in pigment formation. In contrast to the parental strain which produced yellowish-green conidia, the knockout mutants produced white and olive-green conidia, respectively. The system was further refined by restoring the pyrithiamine sensitivity and uracil auxotrophy in the A. flavus transformation recipient with an engineered pyrG marker. The improvement allowed gene manipulation using the reusable pyrG marker as shown by the restoration of laeA-mediated aflatoxin production in an A. flavus laeA-deleted mutant.
Epidemiology of aflatoxin formation by Aspergillus flavus
Aspergillus flavus: human pathogen, allergen and mycotoxin producer
Ste20 and Cla4 modulate the expression of the glycerol biosynthesis enzyme Gpd1 by a novel MAPK-independent pathway
DOI:10.1016/j.bbrc.2019.07.072
URL
PMID:31395335
[本文引用: 2]

p21-activated kinases (PAKs) are important signalling molecules with a wide range of functions. In budding yeast, the main PAKs Ste20 and Cla4 regulate the response to hyperosmotic stress, which is an excellent model for the adaptation to changing environmental conditions. In this pathway, the only known function of Ste20 and Cla4 is the activation of a mitogen-activated protein kinase (MAPK) cascade through Ste11. This eventually leads to increased transcription of glycerol biosynthesis genes, the most important response to hyperosmotic shock. Here, we show that Ste20 and Cla4 not only stimulate transcription, they also bind to the glycerol biosynthesis enzymes Gpd1, Gpp1 and Gpp2. Protein levels of Gpd1, the enzyme that catalyzes the rate limiting step in glycerol synthesis, positively correlate with glucose availability. Using a chemical genetics approach, we find that simultaneous inactivation of STE20 and CLA4 reduces the glucose-induced increase of Gpd1 levels, whereas the deletion of either STE20 or CLA4 alone has no effect. This is also observed for the hyperosmotic stress-induced increase of Gpd1 levels. Importantly, under both conditions the deletion of STE11 has no effect on Gpd1 induction. These observations suggest that Ste20 and Cla4 not only have a role in the transcriptional regulation of GPD1 through Ste11. They also seem to modulate GPD1 expression at another level such as translation or protein degradation.
The identification of Pcl1-interacting proteins that genetically interact with Cla4 may indicate a link between G1 progression and mitotic exit
Identification of p21-activated kinase specificity determinants in budding yeast: a single amino acid substitution imparts Ste20 specificity to Cla4
Cla4 PAK-like kinase is required for pathogenesis, asexual/sexual development and polarized growth in Bipolaris maydis
Aspergillus flavus: the major producer of aflatoxin
Virulence and hyphal formation of Candida albicans require the Ste20p-like protein kinase CaCla4p
DOI:10.1016/s0960-9822(06)00252-1
URL
PMID:9259554
[本文引用: 2]

BACKGROUND: The pathogenic fungus Candida albicans is capable of a morphological transition from a unicellular budding yeast to a filamentous form. Extensive filamentous growth leads to the formation of mycelia displaying hyphae with branches and lateral buds. Hyphae have been observed to adhere to and invade host tissues more readily than the yeast form, suggesting that filamentous growth may contribute to the virulence of this major human pathogen. A molecular and genetic understanding of the potential role of morphological switching in the pathogenicity of C. albicans would be of significant benefit in view of the increasing incidence of candidiasis. RESULTS: The CaCLA4 gene of C. albicans was cloned by functional complementation of the growth defect of cells of the budding yeast Saccharomyces cerevisiae deleted for the STE20 gene and the CLA4 gene. CaCLA4 encodes a member of the Ste20p family of serine/threonine protein kinases and is characterized by a pleckstrin homology domain and a Cdc42p-binding domain in its amino-terminal non-catalytic region. Deletion of both alleles of CaCLA4 in C. albicans caused defects in hyphal formation in vitro, in both synthetic liquid and solid media, and in vivo in a mouse model for systemic candidiasis. The gene deletions reduced colonization of the kidneys in infected mice and suppressed C. albicans virulence in the mouse model. CONCLUSIONS: Our results demonstrate that the function of the CaCla4p protein kinase is essential for virulence and morphological switching of C. albicans in a mouse model. Thus, hyphal formation of C. albicans mediated by CaCla4p may contribute to the pathogenicity of this dimorphic fungus, suggesting that regulators of morphological switching may be useful targets for antifungal drugs.
The PAK family kinase Cla4 is required for budding and morphogenesis in Ustilago maydis
A brain serine/threonine protein kinase activated by Cdc42 and Rac1
MAP kinase kinase kinase, MAP kinase kinase and MAP kinase
Global phosphoproteomic analysis reveals the lnvolvement of phosphorylation in aflatoxins biosynthesis in the pathogenic fungus Aspergillus flavus
DOI:10.1038/srep34078
URL
PMID:27667718
[本文引用: 2]

Aspergillus flavus is a pathogenic fungus that produces toxic and carcinogenic aflatoxins and is the causative agent of aflatoxicosis. A growing body of evidence indicates that reversible phosphorylation plays important roles in regulating diverse functions in this pathogen. However, only a few phosphoproteins of this fungus have been identified, which hampers our understanding of the roles of phosphorylation in A. flavus. So we performed a global and site-specific phosphoproteomic analysis of A. flavus. A total of 598 high-confidence phosphorylation sites were identified in 283 phosphoproteins. The identified phosphoproteins were involved in various biological processes, including signal transduction and aflatoxins biosynthesis. Five identified phosphoproteins associated with MAPK signal transduction and aflatoxins biosynthesis were validated by immunoblotting using phospho-specific antibodies. Further functional studies revealed that phosphorylation of the MAP kinase kinase kinase Ste11 affected aflatoxins biosynthesis in A. flavus. Our data represent the results of the first global survey of protein phosphorylation in A. flavus and reveal previously unappreciated roles for phosphorylation in the regulation of aflatoxins production. The generated dataset can serve as an important resource for the functional analysis of protein phosphorylation in A. flavus and facilitate the elucidation of phosphorylated signaling networks in this pathogen.
The small GTPase Rac and the p21-activated kinase Cla4 in Claviceps purpurea: interaction and impact on polarity, development and pathogenicity
Practice guidelines for diseases caused by Aspergillus
Cla4 protein kinase is essential for filament formation and invasive growth of Yarrowia lipolytica
Adaptor functions of Cdc42, Ste50, and Sho1 in the yeast osmoregulatory HOG MAPK pathway
Small GTPase Rac1 and its interaction partner Cla4 regulate polarized growth and pathogenicity in Verticillium dahliae
Regulation of development in Aspergillus nidulans and Aspergillus fumigatus
Double-joint PCR: a PCR-based molecular tool for gene manipulations in filamentous fungi
DOI:10.1016/j.fgb.2004.08.001
URL
PMID:15465386
[本文引用: 1]

Gene replacement via homologous double crossover in filamentous fungi requires relatively long (preferentially >0.5 kb) flanking regions of the target gene. For this reason, gene replacement cassettes are usually constructed through multiple cloning steps. To facilitate gene function studies in filamentous fungi avoiding tedious cloning steps, we have developed a PCR-assisted DNA assembly procedure and applied it to delete genes in filamentous fungi. While the principle of this procedure is essentially the same as other recently reported PCR-based tools, our technique has been effectively used to delete 31 genes in three fungal species. Moreover, this PCR-based method was used to fuse more than 10 genes to a controllable promoter. In this report, a detailed protocol for this easy to follow procedure and examples of genes deleted or over-expressed are presented. In conjunction with the availability of genome sequences, the application of this technique should facilitate functional characterization of genes in filamentous fungi. To stream line the analysis of the transformants a relatively simple procedure for genomic DNA or total RNA isolation achieving approximately 100 samples/person/day is also presented.
Two new cleistanthane diterpenes and a new isocoumarine from cultures of the Basidiomycete. Albatrellus confluens
DOI:10.1248/cpb.57.975
URL
PMID:19721259
[本文引用: 2]

Two new cleistanthane-type diterpenes, 3alpha,5alpha,8beta-trihydroxycleistanth-13(17),15-dien-18-oic acid (1) and 8beta-hydroxy-18-norcleistanth-4(5),13(17),15-trien-3-one (2), a new isocoumarine, 3R-(2R-hydroxypropyl)-8-hydroxyl-7-methyl-3,4-dihydroisocoumarine (3), along with three known aurovertins, aurovertins B (4), C (5) and E (6), four known polyesters, orbuticin (7), BK223A (8), BK223B (9) and 15G256alpha-2-me (10), and a known isocoumarine, 3R-6-hydroxymellein (11), were isolated from cultures of the basidiomycete Albatrellus confluens. The structures of these compounds were established on the basis of spectroscopic and chemical methods.