通过计算机辅助发现药物的作用靶点,对确定化合物的靶点以及构效关系的研究具有提示作用。其中,分子对接是研究分子间(如配体和受体)相互作用的一种理论模拟方法(Ferreira et al. 2015),当今很多学者通过分子对接技术筛选抗肿瘤靶点和小分子抑制剂(顾勇亮等 2015;王樟根 2017;Chen et al. 2017)。鉴于目前灵芝三萜类化合物的构效关系研究中所选化合物过少、类型不全面的现状,本研究在前期制备了赤芝Ganoderma lingzhi子实体、菌丝体和层迭灵芝Ganoderma lobatum (Cooke) G.F. Atk.子实体中多种类型的羊毛甾烷型三萜化合物的基础上(岳亚文等 2020;彭小芳等 2021),对灵芝酸、烯酸、酮、醛、醇以及环氧酸类三萜进行了体外抗肿瘤活性的比较,总结具有抗肿瘤活性潜力的羊毛甾烷型三萜的结构特征并进一步通过计算机分子对接技术探索其活性位点,为高活性灵芝三萜化合物的寻找和结构改造提供参考。
1 材料与方法
1.1 材料、试剂和仪器
1.1.1 实验材料
45个羊毛甾烷型三萜化合物购自国家标准物质中心或自制,纯度≥98%。L1210:小鼠白血病细胞株,购自中国科学院上海生命科学研究院细胞资源中心。
1.1.2 主要试剂
胎牛血清(fetal bovine serum,FBS)、RPMI1640培养基、1%双抗(青霉素和链霉素)、磷酸缓冲盐溶液(phosphate buffered saline, PBS) (Gibco公司);5-氟尿嘧啶[3H)-pyrimidinedione,5FU]、二甲基亚砜(dimethyl sulfoxide,DMSO) (Sigma公司);Alamar Blue试剂(Biosource公司)。
1.1.3 主要仪器
生物安全柜、二氧化碳培养箱、超低温冰箱(Thermo公司);高压灭菌锅(TOMY公司);恒温水浴锅(上海一恒科技有限公司);多功能倒置荧光显微镜(Olympus公司);多功能酶标仪(Bio-Tek公司);细胞计数仪(Beckman-Coulter公司);台式高速大容量离心机(Eppendorf公司);漩涡混合器(上海精科实业有限公司)。
1.2 方法
1.2.1 样品溶液的制备
将三萜化合物用DMSO配制成20 mg/mL的母液,再用DMSO逐级稀释为1、4和10 mg/mL的样品工作液。将5FU用DMSO配制成10 mg/mL的溶液,作为阳性对照备用。
1.2.2 抗肿瘤活性测定
将L1210细胞置于RPMI 1640 (含1%双抗和10% FBS,下同)中,于5% CO2、37 ℃培养箱内培养传代。取对数生长期的L1210细胞,用RPMI 1640培养基稀释成2×104个/mL细胞悬液,按200 μL/孔接种于96孔板中,再分别加入1 μL样品溶液、阴性对照(DMSO)和阳性对照(5FU),每组设置3个生物重复。将96孔板置于二氧化碳培养箱内培养72 h取出,用多功能酶标仪在570 nm、600 nm处测定其吸光度(A)值作为初始值,然后加入30 μL 0.1 mg/mL的Alamar Blue试剂,再放入培养箱培养4 h左右,取出再次测定其吸光度(A)值。参照冯娜等(2010)的方法,依据增殖抑制率计算公式计算各样品对肿瘤细胞L1210的增殖抑制率:
细胞抑制率(%)=
1.2.3 小分子配体的准备
应用画图软件ChemDraw画出三萜化合物的结构,导入软件Discovery Studio 2016 (DS2016)中。考虑到配体数目、对映异构体和pH等因素,采用DS2016中的Prepare Ligand处理该体系,通过此操作给小分子配体进行加氢,产生三维结构和异构体。导入的12个三萜化合物经处理后产生76个用于对接的配体分子。
1.2.4 蛋白受体的准备
研究所用受体蛋白及功能见表1,其晶体结构均源自RCSB PDB蛋白质数据库(
表1 分子对接所使用的受体
Table 1
| PDB ID | 缩写 Abbreviation | 名称 Name | 功能 Function |
|---|---|---|---|
| 2XOW | p53 | p53蛋白 p53 protein | 防止癌变,修复缺陷 Prevent cancer and repair defects |
| IYSI | Bcl-xl | 抗凋亡蛋白 Anti-apoptotic proteins | 阻止凋亡 Prevent apoptosis |
| 5HG5 | EGFR | 表皮生长因子受体 Epidermal growth factor receptor | 加速促进细胞异常生长和分裂,最终导致肿瘤诞生 Accelerate the abnormal growth and division of cells, and eventually lead to the birth of tumors |
| 1M48 | IL-2 | 白细胞介素-2 Interleukin-2 | 促进淋巴细胞生长、增殖、分化,能诱导和增强细胞毒活性 Promote the growth, proliferation and differentiation of lymphocyte, induce and enhance cytotoxic activity |
| 1Y6B | VEGFR2 | 血管内皮细胞生长因子受体2 Vascular endothelial growth factor receptor 2 | 调节淋巴管内皮细胞和血管内皮细胞,促进淋巴管和血管的生成,还有调节淋巴细胞的迁移等作用 Regulate lymphatic endothelial cells and vascular endothelial cells, promote the production of lymphatic vessels and blood vessels, and regulate the migration of lymphocytes, etc. |
1.2.5 分子对接
以三萜化合物为对接配体,以p53、Bcl-xl、EGFR、IL-2和VEGFR2蛋白为对接受体,利用DS2016软件的LibDock模块进行基于结构的配 体-蛋白分子对接,修改对接参数为Conformation Preferences:User Specified,其他参数均为默认值。对接结果以Libdock Score打分函数列出。Libdock Score越高,预测配体与受体蛋白结合的活性越高。另外通过DS2016软件分析对接结果中的配体与受体蛋白之间相互作用类别,找到配体与氨基酸残基对接的主要活性位点。
2 结果与分析
2.1 三萜化合物的抗肿瘤活性
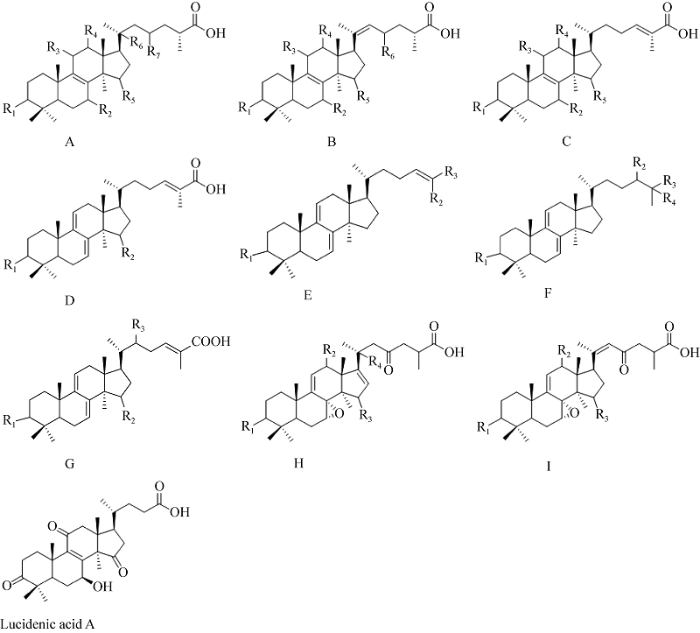
45个三萜化合物的结构通式见图1。根据化合物的来源不同,可分为赤芝子实体酸性三萜、赤芝子实体中性三萜、赤芝菌丝体三萜和层迭灵芝子实体三萜。这些化合物的结构及对小鼠白血病细胞L1210增殖的半数抑制浓度结果分别见表2-表5。阳性对照5FU的增殖抑制率是90.83%。结果表明,在赤芝子实体酸性三萜化合物中,ganoderic acid I、ganoderic acid D的活性较强,其增殖抑制的半数抑制浓度(IC50)值分别为39.54 μmol/L和3.67 μmol/L;其他酸性三萜化合物对L1210的IC50均在50 μmol/L以上;ganoderic acid ε、ganoderenic acid C、ganoderic acid N和ganoderic acid Y在受试浓度范围内甚至未表现出抑制L1210增殖的作用。灵芝子实体中性三萜中,ganoderiol A和ganoderal A的活性较好,其IC50分别为30.30 μmol/L和28.44 μmol/L。灵芝菌丝体中的8个三萜化合物均表现出较强的抑制L1210细胞增殖的能力,IC50均在50 μmol/L以下,其中ganoderic acid T的活性最强,其对L1210的IC50仅为1.92 μmol/L。层迭灵芝中6个树舌环氧酸类三萜化合物对L1210细胞增殖抑制的能力则普遍较差,除applanoxidic acid E外,其他树舌环氧酸类三萜对L1210细胞株的IC50均在150 μmol/L以上。该结论与有关文献报道的结果相吻合(刘如明 2012;唐庆九等 2010;Shao et al. 2020;岳亚文等2020)。
图1
图1
不同类型羊毛甾烷型三萜化合物的结构通式
Fig. 1
General structural formula of lanostane triterpenes with different types.
表2 灵芝子实体酸性三萜化合物对L1210细胞增殖的抑制作用
Table 2
| 灵芝子实体酸性三萜 Acidic triterpenes from fruiting bodies of G. lingzhi | 母环 Female ring | R1 | R2 | R3 | R4 | R5 | R6 | R7 | IC50 (μmol/L) |
|---|---|---|---|---|---|---|---|---|---|
| Ganoderic acid I | A | β-OH | β-OH | =O | -H | =O | β-OH | =O | 39.54 |
| Ganoderic acid ε | A | β-OH | β-OH | =O | -H | =O | -H | β-OH | - |
| Ganoderenic acid C | B | β-OH | β-OH | =O | -H | α-OH | =O | - | - |
| Ganoderic acid C2 | A | β-OH | β-OH | =O | -H | α-OH | -H | =O | 520.54 |
| Ganoderic acid C6 | A | β-OH | =O | =O | β-OH | =O | -H | =O | 2 793.27 |
| Ganoderic acid G | A | β-OH | β-OH | =O | β-OH | =O | -H | =O | 58.26 |
| Ganoderic acid B | A | β-OH | β-OH | =O | -H | =O | -H | =O | 77.32 |
| Ganoderenic acid B | B | β-OH | β-OH | =O | -H | =O | =O | - | 58.81 |
| Ganoderenic acid A | B | =O | β-OH | =O | -H | α-OH | =O | - | 351.85 |
| Ganoderic acid A | A | =O | β-OH | =O | -H | α-OH | -H | =O | 104.19 |
| Ganoderic acid K | A | β-OH | β-OH | =O | β-OAc | =O | -H | =O | 116.97 |
| Ganoderenic acid E | B | =O | β-OH | =O | β-OH | =O | =O | 135.46 | |
| Ganoderic acid H | A | β-OH | =O | =O | β-OH | =O | -H | =O | 417.07 |
| Ganoderenic acid H | B | β-OH | =O | =O | -H | =O | =O | - | 108.75 |
| Lucidenic acid A | - | - | - | - | - | - | - | - | 103.41 |
| Ganoderic acid N | A | =O | β-OH | =O | -H | =O | -H | =O | - |
| Ganoderic acid D | A | =O | β-OH | =O | -H | =O | -H | =O | 3.67 |
| Ganoderenic acid D | B | =O | β-OH | =O | -H | =O | =O | 27 094.48 | |
| Ganoderic acid Z | C | β-OH | =O | =O | -H | -H | 2 289.44 | ||
| Ganoderic acid F | A | =O | =O | =O | β-OAc | =O | -H | =O | 265.35 |
| Ganoderenic acid F | B | =O | =O | =O | -H | =O | =O | - | 72.91 |
| Ganoderic acid DM | C | =O | =O | -H | -H | -H | - | - | 75.48 |
| Ganoderic acid Y | D | β-OH | -H | - | - | - | - | - | - |
| Ganoderic acid TN | D | β-OH | β-OAc | - | - | - | - | - | 57.75 |
注:“-”表示在受试浓度下化合物对L1210无作用,下同
Note: “-” indicates that the compound has no effect on L1210 at the tested concentration, the same below.
表3 灵芝子实体中性三萜化合物对L1210细胞增殖的抑制作用
Table 3
| 灵芝子实体中性三萜 Neutral triterpenes from fruiting bodies of G. lingzhi | 母环 Female ring | R1 | R2 | R3 | R4 | IC50 (μmol/L) |
|---|---|---|---|---|---|---|
| Ganodermanontriol | F | =O | α-OH | -CH2OH | β-OH | 51.05 |
| Ganoderiol A | F | β-OH | -OH | -CH2OH | -OH | 30.30 |
| Ganodermanondiol | F | =O | α-OH | -CH3 | -OH | 137.38 |
| Ganoderiol F | E | =O | -CH2OH | -CH2OH | - | 147.92 |
| Ganoderol A | E | =O | -CH3 | -CH2OH | - | 65.21 |
| Ganoderal A | E | =O | -CH3 | -CHO | - | 28.44 |
| Ganoderol B | E | β-OH | -CH3 | -CH2OH | - | 54.28 |
表4 灵芝菌丝体三萜化合物对L1210细胞增殖的抑制作用
Table 4
| 灵芝菌丝体三萜 Mycelial triterpenes | 母环 Female ring | R1 | R2 | R3 | IC50 (μmol/L) |
|---|---|---|---|---|---|
| Ganoderic acid T | G | α-OAc | α-OAc | β-OAc | 1.92 |
| Ganoderic acid S | G | α-OH | -H | β-OAc | 19.33 |
| Ganoderic acid P | G | α-OH | α-OAc | β-OAc | 26.66 |
| Ganoderic acid T1 | G | α-OAc | α-OAc | β-OH | 21.12 |
| Ganoderic acid Mk | G | α-OAc | α-OH | β-OAc | 16.71 |
| Ganoderic acid Me | G | α-OAc | α-OAc | -H | 9.66 |
| Lanosta-7,9(11),24-trien-3α-hydroxy-26-oic acid | G | α-OH | -H | -H | 29.97 |
| Ganoderic acid R | G | α-OAc | -H | β-OAc | 31.69 |
表5 层迭树舌子实体三萜化合物对L1210细胞增殖的抑制作用
Table 5
| 树舌环氧酸三萜 Applanoxidic acids | 母环 Female ring | R1 | R2 | R3 | R4 | IC50 (μmol/L) |
|---|---|---|---|---|---|---|
| Applanoxidic acid H | H | β-OH | α-OH | =O | -OH | 10 083.46 |
| Applanoxidic acid A | I | =O | =O | α-OH | - | 272.52 |
| Applanoxidic acid G | H | =O | =O | β-OH | -OH | 3 477.67 |
| Applanoxidic acid C | H | =O | =O | =O | -OH | 1 507.38 |
| Applanoxidic acid E | I | =O | =O | β-OH | - | 84.64 |
| Applanoxidic acid F | I | =O | =O | =O | - | 166.01 |
2.2 三萜化合物与蛋白对接
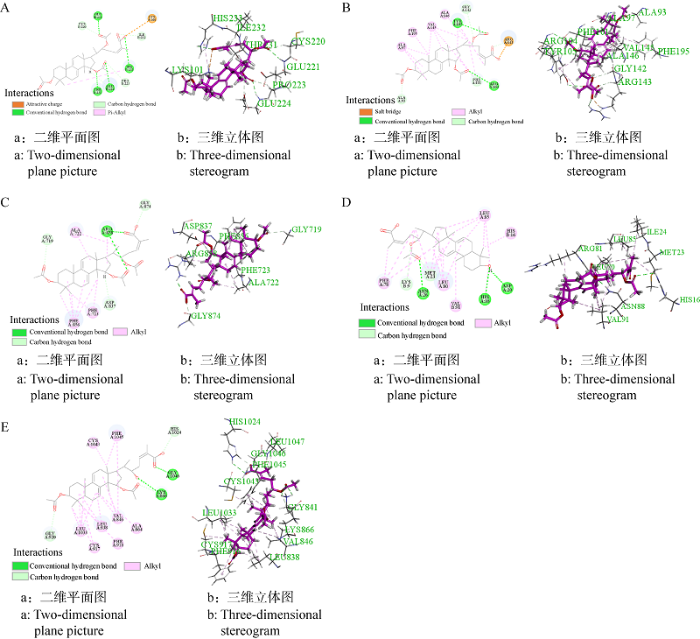
p53、Bcl-xl、EGFR、IL-2和VEGFR2均是与抗肿瘤作用相关的靶点蛋白,选择这些蛋白作为受体,与实验结果中IC50值小于50 μmol/L的12个三萜化合物产生的76个配体进行分子对接。对接Poses以及LibDock打分结果见 表6。由对接结果可知,除ganoderic acid Mk、ganoderic acid_I、ganoderic acid D不能与靶蛋白EGFR对接外,其他三萜化合物均可以与5种靶点蛋白对接,说明灵芝三萜抑制肿瘤的过程中存在多靶点作用。在这些化合物的对接模拟中,灵芝菌丝体来源的三萜均表现优异。其中,ganoderic acid T的表现最好,其与靶点蛋白p53和Bcl-xl对接位点的数目及对接得分均排在第一位,表明ganoderic acid T与抗肿瘤作用靶点有较强的结合能力。为进一步观察配体分子与靶蛋白发生的相互作用,将每个靶蛋白对应打分最高的配体的对接结果以二维平面图展示出来做进一步分析。
表6 配体与靶蛋白对接
Table 6
| 配体 Ligand | p53 | Bcl-xl | EGFR | IL-2 | VEGFR2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Poses | Score | Poses | Score | Poses | Score | Poses | Score | Poses | Score | |
| Ganoderic acid P | 10 | 115.52 | 10 | 142.42 | 5 | 123.16 | 5 | 118.39 | 10 | 133.25 |
| Ganoderma acid T1 | 3 | 106.89 | 10 | 140.52 | 22 | 111.43 | 1 | 102.88 | 10 | 135.49 |
| Ganoderic acid S | 6 | 113.64 | 10 | 131.19 | 3 | 119.55 | 10 | 145.95 | 10 | 123.33 |
| Ganoderic acid T | 10 | 119.76 | 10 | 152.44 | 4 | 132.70 | 2 | 116.23 | 10 | 126.43 |
| Ganoderic acid Me | 10 | 112.97 | 10 | 141.92 | 3 | 105.81 | 10 | 115.93 | 10 | 131.55 |
| Ganoderic acid R | 10 | 112.53 | 10 | 142.54 | 2 | 121.23 | 10 | 142.28 | 10 | 125.44 |
| Ganoderic acid Mk | 2 | 104.29 | 10 | 136.51 | - | - | 9 | 127.94 | 10 | 127.44 |
| Lanosta-7,9(11),24-trien-3α-hydroxy-26-oic acid | 2 | 108.04 | 10 | 129.37 | 2 | 114.60 | 10 | 131.04 | 10 | 118.92 |
| Ganoderic acid I | 2 | 113.87 | 10 | 134.51 | - | - | 9 | 119.06 | 5 | 105.76 |
| Ganoderic acid D | 2 | 106.48 | 10 | 140.06 | - | - | 10 | 123.40 | 10 | 111.06 |
| Ganoderiol A | 1 | 101.96 | 10 | 141.78 | 2 | 122.61 | 10 | 122.90 | 10 | 116.80 |
| Ganodera A | 1 | 110.40 | 10 | 129.12 | 3 | 104.15 | 10 | 129.88 | 10 | 107.94 |
注:化合物的众多对接Poses中只给出LibDock打分最高值
Note: Only the highest LibDock score is given among many poses.
Ganoderic acid T与Bcl-xl的对接打分最高(表6),分析其结果(图2B)发现,靶点蛋白Bcl-xl上的氨基酸残基与ganoderic acid T的乙酰基和末端羧基存在氢键相互作用,包括ALA93与C-3位上的羰基氧原子,PHE101、ARG104与C-15位上的羰基氧原子,TYR105与C-22位上羰基氧原子,GLY142与C-22位上酯基氧原子,ARG143与末端羟基氧原子、羰基氧原子。氨基酸残基ALA97、PH195、VAL145、ALA146还与ganoderic acid T之间存在多个π键-烷基化和烷基化作用。此外,氨基酸残基ARG143还与该化合物侧链末端羧基形成盐桥相互作用。由于众多氨基酸残基与ganoderic acid T的相互作用,使得ganoderic acid T配体与受体蛋白的结合活性最强。
图2
图2
灵芝羊毛甾烷型三萜与靶蛋白分子对接
A:Ganoderic acid T与p53蛋白对接;B:Ganoderic acid T与Bcl-xl蛋白对接;C:Ganoderic acid T与EGFR蛋白对接;D:Ganoderic acid S与IL-2蛋白对接;E:Ganoderic acid T1与VEGFR2蛋白对接
Fig. 2
Molecular docking of lanostane triterpenes from Ganoderma spp. to target proteins.
A: Docking of ganoderic acid T to p53 protein; B: Docking of ganoderic acid T to Bcl-xl protein; C: Docking of ganoderic acid T to EGFR protein; D: Docking of ganoderic acid S to IL-2 protein; E: Docking of ganoderic acid T1 to VEGFR2 protein.
3 讨论
刘如明(2012)通过研究ganoderic acid T、Mk、T1和T2 4种灵芝酸对Hela细胞的细胞毒性及诱导细胞凋亡的能力发现,乙酰化程度越高的灵芝酸,其细胞毒性及诱导肿瘤细胞凋亡的能力越强,并且ganoderic acid T可以通过靶向p53蛋白抑制癌细胞侵袭、增殖(Chen & Zhong 2011;唐文等 2015)。本研究体外活性实验结果表明,具有3个乙酰氧基的ganoderic acid T对L1210细胞毒性最强,具有2个乙酰氧基的ganoderic acid P次之,只有1个乙酰氧基的ganoderic acid S较弱,这一结果与此前的研究结论一致。本研究进一步通过计算机虚拟对接的方式,将对L1210细胞增殖抑制能力较强的12个灵芝三萜化合物与p53、Bcl-xl、EGFR、IL-2和VEGFR2 (表达异常时会促进肿瘤增殖、迁移)抗肿瘤靶蛋白进行分子对接,结果显示灵芝羊毛甾烷型三萜化合物结构中的乙酰基、羟基、羧基可以与受体蛋白活性口袋里的氨基酸残基之间形成氢键、烷基化(疏水作用)等相互作用,证明了乙酰基、羟基以及末端羧基是该类三萜化合物发挥药效的重要官能团。本研究在定义对接活性位点时选择了From receptor cavities这一选项,因此推测当灵芝三萜化合物与受体腔中的氨基酸残基存在相互作用时,这些三萜化合物对靶蛋白的抑制类型是竞争性抑制。此外,分子对接的结果显示,三萜化合物与氨基酸残基主要通过氢键、具有疏水作用的烷基化等非共价键来发生作用,由此,推测这些三萜化合物对靶蛋白的抑制作用是可逆的。
有研究认为,ganoderic acid A 和ganoderic acid H通过抑制转录因子AP-1和NF-κB从而抑制乳腺癌细胞MDA-MB-231的生长与侵袭行为,推测这一活性与三萜羊毛甾烷结构中C-3、C-7和C-15位上的羟基有关(Jiang et al. 2008)。本研究的体外实验结果表明,在以上3个位点有羟基的化合物,例如ganoderic acid ε、ganoderenic acid C和ganoderic acid N,对小鼠白血病细胞L1210增殖抑制的能力并不显著。因此,关于C-3、C-7和C-15位上羟基的构效关系值得继续探讨。
本研究通过细胞增殖抑制率测定实验对比研究发现,子实体中的三萜醇ganodermanontriol、ganoderal A和ganoderiol A等同菌丝体中的三萜酸ganoderic acid T、ganoderic acid T1和ganoderic acid P一样,母环上都具有△7,8、△9,11共轭双键且抗肿瘤活性突出,因此母环上共轭双键的存在可能有助于提高化合物的抗肿瘤活性。Cheng et al. (2010)研究结果也表明,双键位于7位和9位的化合物比具有7-酮-8-烯结构的化合物表现出更高的活性。此外,这些活性突出的三萜醇类化合物,支链末端的羟基是否与其活性有关,值得进一步探讨。
参考文献
P 53 is important for the anti-invasion of ganoderic acid T in human carcinoma cells
DOI:10.1016/j.phymed.2011.01.011 URL [本文引用: 1]
Chinese edible medicinal fungal chemistry
Cytotoxic lanostane-type triterpenoids from the fruiting bodies of Ganoderma lucidum and their structure-activity relationships
DOI:10.18632/oncotarget.14336 URL [本文引用: 1]
Cytotoxic triterpenoids from Ganoderma lucidum
DOI:10.1016/j.phytochem.2010.06.005 URL [本文引用: 1]
The scientific name of the widely cultivated Ganoderma species
Diversity and systematics of the important macrofungi in Chinese forests
Anti-tumor target prediction and activity verification of Ganoderma lucidum triterpenoids
Steroids from fruiting bodies of Coprinus comatus and their inhibition to tumor cell proliferation
Molecular docking and structure-based drug design strategies
DOI:10.3390/molecules200713384 URL [本文引用: 1]
Molecular dynamics simulation of binding of cyanopyrrolidine inhibitors to dipeptidyl peptidase-4 (DPP-4)
Ganoderic acids suppress growth and invasive behavior of breast cancer cells by modulating AP-1 and NF-κB signaling
Molecular modelling study on structure and function of protein kinase A and its inhibitor
Modern research of Ganoderma. 4th ed
Ganoderma and health: biology, chemistry and industry
Structure modification of ganoderic acids and study on anti-tumor mechanism of novel ganoderic acid derivatives
Cytotoxic and pro-apoptotic effects of novel ganoderic acid derivatives on human cervical cancer cells in vitro
DOI:10.1016/j.ejphar.2012.02.007 URL [本文引用: 1]
Triterpenoids from the spores of Ganoderma lucidum
Preparation of ganodermenonol by high speed countercurrent chromatography
Ganoderic acid D induces synergistic autophagic cell death except for apoptosis in ESCC cells
DOI:10.1016/j.jep.2020.113213 URL [本文引用: 1]
Inhibition of tumor cell proliferation by a neutral triterpenoid fraction from Ganoderma lucidum
Inhibition of ganoderic acid mediated by P 53 protein on cancer cells proliferation
Studies on the active constituents of Ganoderma lucidum against human breast cancer cells and their structure-activity relationships
Species diversity, taxonomy and phylogeny of Ganoderma
Difference of chemical components in fruiting body, mycelium and spore powder of Ganoderma lingzhi
Active components in mycelia of sporeless Ganoderma lingzhi
中国森林大型真菌重要类群多样性和系统学研究
毛头鬼伞子实体中甾类化合物的结构鉴定及其抑制肿瘤细胞增殖活性的研究