灵芝Ganoderma lingzhi Sheng H. Wu, Y. Cao & Y.C. Dai属多孔菌目灵芝科灵芝属(Cui et al. 2019;亓小妮等 2021),其主要活性成分灵芝三萜类化合物具有广泛的药理活性,具有抗肿瘤(唐庆九等 2010)、保肝(王明宇等2000)、抗HIV-1(Gao et al. 2003)、免疫调节(Feng et al. 2013)、降血脂(衣艳君和徐承水 2001)、抗真菌(Vazirian et al. 2014)、抗炎(Shailesh et al. 2009)和抗氧化(Hsu et al. 2018;Wu et al. 2019)等作用,因此对灵芝三萜的研究有着十分重要的意义。
灵芝三萜的主要来源是通过栽培方式的灵芝子实体及孢子粉和液态深层发酵方式的菌丝体及胞外液获得。与传统栽培相比,液态深层发酵方法有发酵周期短、生长速度快,发酵条件更易控制等优点(陈慧等 2015)。现阶段,通过液态深层发酵方式获取灵芝三萜已经成为研究热点。
近年来,优化灵芝液态深层发酵的工艺提高灵芝三萜等目标产物一般通过培养基配方和培养过程中的参数进行调节,但只能通过外在调控促进灵芝三萜的增长,具有一定的局限性,主要体现在目标产物的提高幅度有限,重复性研究工作较多,优化出的配方成本较高,与实际应用需求差距较大,缺少有意义的创造性研究工作。而在发酵体系中通过添加外源物促进灵芝三萜合成代谢方面的研究逐渐增加(陈慧等 2015)。油脂物质作为其中一类外源添加物,在食用真菌发酵时被利用,为合成次级代谢产物提供更多的能量;其可以通过改变细胞膜通透性和结构或直接影响代谢途径中某些关键酶活性从而促进灵芝的生长代谢(韦朝阳等 2015)。黎李平(2017)研究发现添加主要成分为49.26%油酸、17.83%棕榈酸、31.94%亚油酸和少量硬脂酸的薏苡仁油可以促进灵芝三萜的合成,说明油脂物质可以促进灵芝三萜的合成,但缺少对薏苡仁油中何种脂肪酸起到促进作用的研究。姚强等(2010)发现通过添加硬脂酸、亚油酸和棕榈酸进行灵芝液态深层发酵,不同的脂肪酸对菌丝体的生长和灵芝三萜有不同的影响,Yang et al.(2000)猜测脂肪酸的促进或者抑制作用可能与其碳链的长短以及不饱和程度的差异有关。朱会霞(2013)在比较油酸、α-萘乙酸、L-谷氨酸对灵芝液态深层发酵的影响中,发现影响灵芝三萜生成的最显著因素为油酸;本研究室前期也通过添加6种脂肪酸和表面活性剂类的外源性物质(Feng et al. 2017)进行灵芝液态深层发酵并发现油酸对灵芝菌丝体生长和三萜含量的提高效果最好。但油酸添加浓度过高、未系统研究油酸的添加工艺,且缺少规模化研究。
油酸作为安全且价格较低的单不饱和脂肪酸(冯杰等 2014),是一种很好的促进灵芝三萜产生的外源添加物,其工艺有应用于工业生产的价值。本研究通过单因素实验和响应面实验对不同添加方式的油酸促进灵芝三萜液态深层发酵的工艺进行优化,且通过3L发酵罐验证,为将该工艺应用于灵芝三萜的工业化生产提供前期研究。此外,本研究所用灵芝是中国广泛栽培灵芝,其拉丁学名为Ganoderma lingzhi,该种与过去文献中所述的欧洲灵芝Ganoderma lucidum是不同种类(Cao et al. 2012;戴玉成等 2013)。
1 材料与方法
1.1 供试材料
1.1.1 供试菌株:由中国微生物菌种保藏管理委员会农业微生物中心上海食用菌分中心提供,菌株编号:Ganoderma lingzhi G0023。
1.1.2 试剂:齐墩果酸标准品购自Sigma公司,油酸、香草醛、冰醋酸、高氯酸等分析纯均购自国药集团化学试剂有限公司。
1.1.3 培养基:(1)平板培养基:称取39g PDA培养基(美国BD公司)溶于1 000mL去离子水中,121℃灭菌15min备用。(2)种子液及发酵培养基:20g/L葡萄糖,3g/L酵母粉,1g/L磷酸二氢钾(KH2PO4),1g/L七水硫酸镁(MgSO4·7H2O),121℃灭菌30min备用。
1.2 方法
1.2.1 菌种活化以及种子液制备:挑取斜面保藏的菌种于平板上进行活化,再将平板转接至装有100mL发酵培养基的250mL三角瓶中,摇床(上海智城分析仪器制造有限公司)搅拌转速150r/min,培养温度26℃,培养7d后备用。
1.2.2 发酵摇瓶培养:将制备好的种子液以10%(V/V)接种量接种于发酵培养基中。摇瓶装液量分别为100mL/250mL三角瓶和400mL/1 000mL三角瓶,搅拌转速150r/min、26℃条件下培养 7d。
1.2.3 发酵罐培养:将种子液以10%(V/V)接种量接种于3L发酵罐(上海保兴生物设备工程有限公司)中进行培养。其中,发酵罐装液量2L、搅拌转速100r/min,培养温度26℃、通气量180L/h、培养7d。
1.2.4 油酸添加方式实验:油酸添加方式包括如下两种:一种和培养基混合在一起进行高温灭菌,灭菌条件为121℃、30min;另一种采用0.22μm微孔滤膜进行过滤除菌后再加入培养基中。
1.2.5 油酸添加浓度实验:在发酵培养基中将种子液以10%接种量接入,在第0天分别添加0.5%、1%、1.5%、2%、2.5%、3%、3.5%(V/V)浓度的油酸,培养7d后分别测定灵芝三萜含量和菌丝体生物量。实验重复3次,取平均值。
1.2.6 油酸添加时间实验:在发酵培养基中将种子液以10%接种量接入,分别在第0、1、2、3、4、5、6天添加0.5%(V/V)浓度的油酸,培养7d后分别测定灵芝三萜含量和菌丝体生物量。实验重复3次,取平均值。
1.2.7 油酸添加的响应面实验:在单因素实验的基础上,采用响应面分析方法对所得的发酵条件进一步优化,根据CCD(central composite design)中心组合设计法,将油酸添加的浓度和时间作为实验因素,将灵芝三萜含量作为响应值,进行2因素5水平的响应面实验。实验因素和水平设计见表1。将响应面结果与单因素的实验结果结合分析,确定对灵芝三萜含量影响的最优条件,并在250mL及1 000mL摇瓶和3L发酵罐对优化结果进行验证。
表1 响应面实验因素水平表
Table 1
| 因素 Factors | 代码 Code | 水平Levels | ||||
|---|---|---|---|---|---|---|
| -a | -1 | 0 | +1 | +a | ||
| 时间 Fermentation time (h) | A | 0 | 7 | 24 | 41 | 48 |
| 浓度 Concentration of oleic acid (%, V/V) | B | 0.5 | 0.65 | 1 | 1.35 | 1.5 |
1.2.8 灵芝菌丝体生物量测定(翟双星等 2018):将发酵液在12 000×g离心20min,去除上清液,沉淀用去离子水洗涤3次,收集菌丝体沉淀,于60℃下干燥后称重,结果以g/L表示,即每升发酵液中含有的菌丝体干质量数。
1.2.9 灵芝三萜检测:(1)比色法:将干燥后的菌丝体以料液比1:50使用95%乙醇提取,超声1h,8 000×g离心5min,取上清液,采用香草醛-冰醋酸法(谭洪升等 2018)检测。(2)高效液相色谱法(杨志空等 2020):将干燥后的菌丝体以料液比1:20使用无水乙醇提取,超声1h,8 000×g离心5min,取上清液进行检测。选用Waters 600-717-2996高效液相色谱仪(Waters公司)、Agilent ZORBAX Eclipse C18(4.6mm×250mm,5μm)色谱柱,以乙腈-醋酸(0.01%)水溶液作为流动相进行洗脱,流速为1.0mL/min,柱温30℃,进样量10μL,分析波长为240nm。
1.2.10 数据处理:实验数据采用Microsoft Excel 2013进行统计与分析,采用Design Expert 12进行响应面实验设计和解析,采用Origin 8.5软件进行绘图。
2 结果与分析
2.1 油酸添加单因素实验
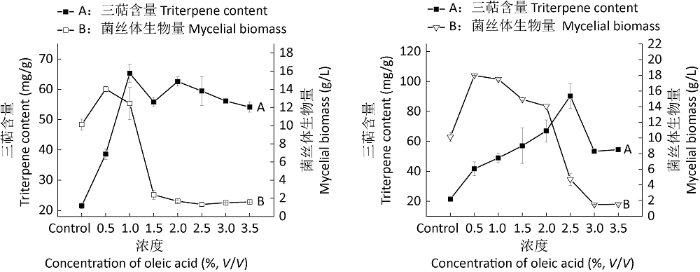
2.1.1 油酸添加浓度实验:分别在第0天添加0.5%-3.5%的油酸,对三萜含量和菌丝体生物量均有不同的影响(图1)。当添加1%灭菌油酸时,三萜含量达到最高,为65.32mg/g,比对照提高了3.04倍;当添加2.5%过滤除菌油酸时,三萜含量达到最高,为90.28mg/g,比对照提高了4.20倍。当添加0.5%灭菌油酸和过滤除菌油酸时,菌丝体生物量达到最大,分别为14.03g/L和17.98g/L,比对照提高了1.38倍和1.77倍。在低浓度时,灵芝三萜含量提高且促进菌丝体的生长,高浓度时灵芝三萜含量和菌丝体生物量皆出现下降趋势,表明分别在添加0.5%-1.0%灭菌油酸和0.5%-2.0%过滤除菌油酸时,最适宜灵芝液态深层发酵。
图1
图1
不同浓度的高温灭菌油酸(左)和过滤除菌油酸(右)对三萜含量和菌丝体生物量的影响
Fig. 1
Effects of different concentrations of moist heat sterilized oleic acid (left) and filtered sterilized oleic acid (right) on triterpene content and mycelial biomass of Ganoderma lingzhi.
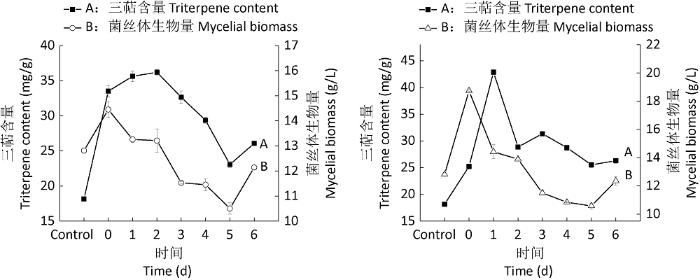
2.1.2 油酸添加时间实验:分别在0-6d添加0.5%油酸,对三萜含量和菌丝体生物量均有不同的影响(图2)。在第2天添加高温灭菌油酸时,三萜含量达到最高,为36.20mg/g,比对照提高了1.99倍;在第1天添加过滤除菌油酸时,三萜含量达到最高,为42.86mg/g,比对照提高了2.36倍。在第0天分别添加高温灭菌油酸和过滤除菌油酸时,菌丝体生物量达到最大,为14.45g/L和18.39g/L,比对照提高了1.18倍和1.44倍。在第6天添加油酸,菌丝体生物量有所上升,这可能与灵芝液态深层发酵达到稳定期、油酸并未能够参与其生长有关。发酵前期,灵芝三萜含量较对照提高且促进菌丝体的生长;发酵后期,灵芝三萜含量和菌丝体生物量皆出现下降趋势,表明分别在第0-2天添加高温灭菌油酸和第0-1天添加过滤除菌油酸,最适宜灵芝液态深层发酵。
图2
图2
不同时间添加高温灭菌油酸(左)和过滤除菌油酸(右)对三萜含量和菌丝体生物量的影响
Fig. 2
Effects of adding moist heat sterilized oleic acid (left) and filtered sterilized oleic acid (right) at different time on triterpene content and mycelial biomass of Ganoderma lingzhi.
2.2 油酸添加响应面实验
在单因素实验的基础上,以灵芝三萜含量为指标,分别添加高温灭菌油酸和过滤除菌油酸进行实验,选取添加时间(h)、添加浓度(%)作为因素,进行2因素5水平响应面分析(表2)。
表2 添加高温灭菌油酸和过滤除菌两种方式下的响应面实验方案及结果
Table 2
| 试验点 Run No. | 时间 Time (h) | 浓度 Concentration (%, V/V) | 高温灭菌油酸条件下三萜含量 Triterpene content under the condition of adding moist heat sterilized oleic acid (mg/g) | 过滤除菌油酸条件下三萜含量 Triterpene content under the condition of adding filtered sterilized oleic acid (mg/g) |
|---|---|---|---|---|
| 1 | 0 | 1 | 33.35±0.88 | 40.41±1.75 |
| 2 | 7 | 1.35 | 40.91±1.38 | 45.74±0.09 |
| 3 | 7 | 0.65 | 26.21±2.28 | 31.64±1.16 |
| 4 | 24 | 1 | 42.28±1.50 | 40.35±1.50 |
| 5 | 24 | 0.5 | 31.25±1.31 | 35.31±0.69 |
| 6 | 24 | 1 | 41.49±1.35 | 43.36±0.75 |
| 7 | 24 | 1.5 | 43.70±1.19 | 47.21±1.37 |
| 8 | 24 | 1 | 47.80±0.50 | 42.08±1.85 |
| 9 | 41 | 1.35 | 45.53±0.47 | 44.59±0.34 |
| 10 | 41 | 0.65 | 42.23±1.63 | 37.12±0.22 |
| 11 | 48 | 1 | 36.56±1.79 | 43.39±1.09 |
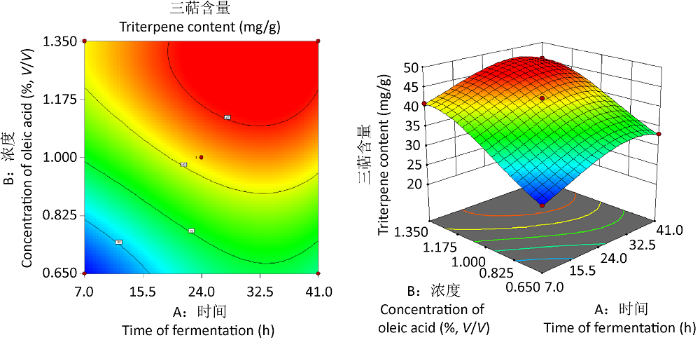
2.2.1 响应面优化高温灭菌油酸添加实验:通过对实验设计结果进行多项式回归拟合分析,所得灵芝三萜含量的优化拟合方程为y=41.52+4.68A+9.10B-0.601AB-3.22A²-1.96B²-1.77A³-2.35B³。
以三萜含量为评价指标的模型P<0.01说明模型具有显著性,该方法可靠;失拟项P=0.6798>0.05,表明失拟项不显著,说明模型计算结果与实际结果差异不显著。本实验相关系数模型R2=0.9966,表明三萜含量的实验值和预测值有很好的一致性,三萜含量回归模型拟合程度较好。从回归系数的显著性检验可知,回归模型中的A、B、A²、B²(P<0.01)皆达到极显著水平(表3),说明添加时间和添加浓度对三萜含量有显著影响。
表3 响应面方差分析结果
Table 3
| 来源 Source | 平方和 Sum of squares | 自由度 Df | 均方 Mean square | F value | P value |
|---|---|---|---|---|---|
| 模型Model | 365.01 | 7 | 52.14 | 123.94 | 0.0011 |
| A | 17.53 | 1 | 17.53 | 41.67 | 0.0075 |
| B | 66.26 | 1 | 66.26 | 157.49 | 0.0011 |
| AB | 1.44 | 1 | 1.44 | 3.43 | 0.1609 |
| A² | 58.47 | 1 | 58.47 | 138.98 | 0.0013 |
| B² | 21.62 | 1 | 21.62 | 51.40 | 0.0056 |
| A3 | 6.28 | 1 | 6.28 | 14.93 | 0.0306 |
| B3 | 11.05 | 1 | 11.05 | 26.25 | 0.0144 |
| 残差Residual | 1.26 | 3 | 0.4207 | ||
| 失拟项 Lack of fit | 0.1294 | 1 | 0.1294 | 0.2285 | 0.6798 |
| 误差 Pure error | 1.13 | 2 | 0.5664 | ||
| 总误差 Cor total | 366.27 | 10 | |||
| R2 | 0.9966 | R2Adj | 0.9885 |
图3
图3
主要因素交互作用的响应曲面图
Fig. 3
Response surface plots showing main factor interaction.
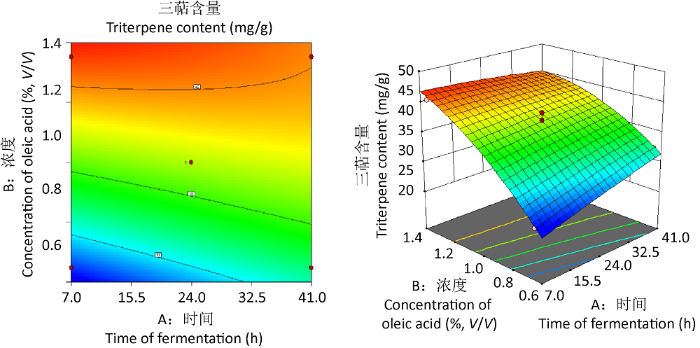
通过响应面实验可得到最优条件为添加时间31.79h、添加高温灭菌油酸浓度1.21%时,预测三萜含量可达到45.63mg/g。根据实验实际可行性,将工艺优化为添加时间32h、添加浓度1.21%进行后续验证。2.2.2 响应面优化过滤除菌油酸添加实验:通过对实验设计结果做多项式回归拟合分析,所得灵芝三萜含量的优化拟合方程为y=41.93+ 1.07A+5.51B-1.66AB-0.2175A²-1.54B²。以三萜含量为评价指标的模型P<0.001说明模型极显著水平,该模型可靠;失拟项P=0.8839>0.05,表明失拟项不显著,说明模型计算结果与实际结果差异不显著。本实验相关系数模型R2=0.9788,表明三萜含量的实验值和预测值有较好的一致性,回归模型拟合程度较好。从回归系数的显著性检验可知,回归模型中的A(P<0.05)较显著水平、B(P<0.001)达到极显著水平(表4),说明添加浓度对三萜含量影响更大。
表4 响应面方差分析结果
Table 4
| 来源 Source | 平方和 Sum of squares | 自由度 Df | 均方 Mean square | F value | P value |
|---|---|---|---|---|---|
| 模型Model | 276.33 | 5 | 55.27 | 46.19 | 0.0003 |
| A | 9.14 | 1 | 9.14 | 7.64 | 0.0397 |
| B | 242.50 | 1 | 242.50 | 202.67 | < 0.0001 |
| AB | 11.01 | 1 | 11.01 | 9.20 | 0.0290 |
| A² | 0.2672 | 1 | 0.2672 | 0.2233 | 0.6564 |
| B² | 13.35 | 1 | 13.35 | 11.16 | 0.0205 |
| 残差Residual | 5.98 | 5 | 1.20 | ||
| 失拟项 Lack of fit | 1.42 | 3 | 0.4747 | 0.2083 | 0.8839 |
| 误差 Pure error | 4.56 | 2 | 2.28 | ||
| 总误差 Cor total | 282.31 | 10 | |||
| R2 | 0.9788 | R2Adj | 0.9576 |
图4
图4
主要因素交互作用的响应曲面图
Fig. 4
Response surface plots showing main factor interaction.
通过响应面实验可得到最佳优化条件为当过滤除菌油酸添加时间为7.03h,添加浓度为1.35%时,预测三萜含量可达到46.27mg/g。根据实验的实际可行性,将工艺优化为添加时间7h、添加浓度1.35%进行验证。
2.3 响应面实验最优条件的摇瓶验证
在250mL摇瓶规模下对响应面最优条件进行验证,结果表明第32h添加1.21%的高温灭菌油酸,灵芝三萜含量达到42.69mg/g,与对照相比提高了2.04倍,比单因素组分别提高了13%和41%;第7h添加1.35%的过滤除菌油酸下灵芝三萜含量达到43.38mg/g,与对照相比提高了2.08倍,比单因素组分别提高了14%和53%(表5)。该验证的实际三萜含量与模型组预测值接近,表明模型预测值和验证值有良好的拟合性,优化模型可靠。
表5 250mL摇瓶规模的响应面摇瓶验证
Table 5
| 类别 Category | 高温灭菌 Moist heat sterilization | 过滤除菌 Filtration sterilization | |||
|---|---|---|---|---|---|
| 三萜含量 Triterpene content (mg/g) | 菌丝体生物量 Mycelial biomass (g/L) | 三萜含量 Triterpene content (mg/g) | 菌丝体生物量 Mycelial biomass (g/L) | ||
| 对照组 Control | 20.89±0.30 | 10.12±0.78 | 20.89±0.30 | 10.12±0.78 | |
| 响应面优化组 Optimal response surface group | 42.69±0.19 | 14.42±0.06 | 43.38±0.30 | 20.79±0.35 | |
| 响应面中心组 Response surface center group | 39.38±0.10 | 13.50±0.59 | 38.78±0.04 | 14.30±0.33 | |
| 单因素优化组 Optimal single factor group | 浓度 Concentration of oleic acid | 37.65±1.62 | 13.89±0.19 | 37.96±0.61 | 19.03±0.16 |
| 时间 Time of fermentation | 30.08±0.18 | 13.69±0.75 | 28.27±0.58 | 14.19±0.97 | |
2.4 放大规模培养验证
在响应面最优条件下放大规模培养,结果显示,第32h添加1.21%的高温灭菌油酸,1 000mL规模下灵芝三萜含量为32.18mg/g,为对照的1.96倍;3L发酵罐规模下灵芝三萜含量为28.66mg/g,是对照的1.62倍(表6)。第7h添加1.35%的过滤除菌油酸,1 000mL规模下灵芝三萜含量32.48mg/g,为对照的1.95倍;3L发酵罐规模下灵芝三萜含量为25.13mg/g,为对照的1.42倍。
表6 放大规模的响应面验证
Table 6
| 规模 Scale | 类别 Category | 高温灭菌 Moist heat sterilization | 过滤除菌 Filtration sterilization | ||
|---|---|---|---|---|---|
| 三萜含量 Triterpene content (mg/g) | 菌丝体干重 Mycelial dry weight (g/L) | 三萜含量 Triterpene content (mg/g) | 菌丝体干重 Mycelial dry weight (g/L) | ||
| 1 000mL摇瓶 1 000mL shaking flask | 对照组 Control | 16.62±0.22 | 7.22±0.12 | 16.62±0.22 | 7.22±0.12 |
| 响应面优化组 Optimal response surface group | 32.18±0.89 | 13.63±0.14 | 32.43±0.17 | 14.01±0.01 | |
| 响应面中心组 Response surface center group | 29.84±0.85 | 11.64±0.11 | 32.35±0.87 | 12.78±0.06 | |
| 3L发酵罐 3L fermenter | 对照组 Control | 17.67±0.29 | 7.76±0.15 | 17.67±0.29 | 7.76±0.15 |
| 响应面优化组 Optimal response surface group | 28.66±0.44 | 11.67±0.29 | 25.13±0.47 | 13.20±0.04 | |
2.5 优化条件下3L发酵罐培养过程分析及灵芝三萜检测分析
图5
图5
不同添加方式的油酸的菌丝体生物量(左)和三萜含量(右)的变化
Fig. 5
Changes in mycelial biomass (left) and triterpene content (right) of fermentation broth with additional moist heat sterilized and filtered sterilized oleic acid.
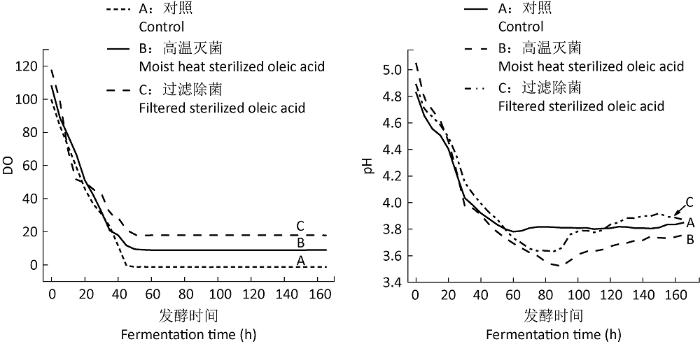
在灵芝液态深层发酵过程中对其进行监测溶氧量(dissolved oxygen,DO)和pH的变化可知,DO随着发酵时间的增长而逐渐降低(图6)。pH随着发酵时间的增长而下降,到后期皆呈平缓趋势。在发酵中期,油酸未被完全利用,油酸作为一种酸性的不饱和脂肪酸,加上次级代谢产物的增加使得pH下降;后期油酸逐渐被利用,则pH缓慢上升且趋于平缓。
图6
图6
两种添加方式油酸发酵液DO(左)和pH(右)的变化
Fig. 6
Changes in DO (left) and pH (right) of fermentation broth with moist heat sterilized and filtered sterilized oleic acid.
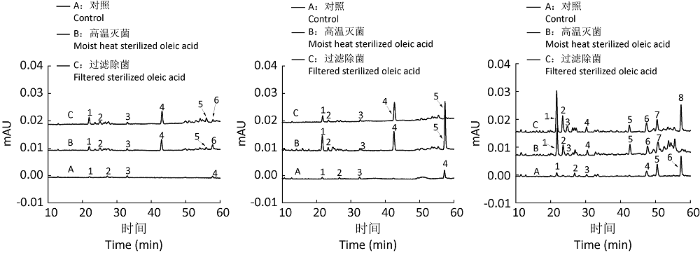
图7
图7
250mL摇瓶(左)、1 000mL摇瓶(中)和3L发酵罐(右)规模的灵芝三萜HPLC图
Fig. 7
HPLC fingerprint of fermented Ganoderma triterpenes in the scales of 250mL shaking flask (left), 1 000mL shaking flask (medium) and 3L fermenters (right) under the condition of adding moist heat sterilized and filtered sterilized oleic acid.
表7 不同发酵规模下的灵芝三萜HPLC图的峰面积
Table 7
| 规模 Scale | 250mL摇瓶 250mL shaking flask | 1 000mL摇瓶 1 000mL shaking flask | 3L发酵罐 3L fermenter | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 名称 Name | 对照 Control | 高温灭菌 Moist heat sterilized oleic acid | 过滤除菌 Filtered sterilized oleic acid | 对照 Control | 高温灭菌 Moist heat sterilized oleic acid | 过滤除菌 Filtered sterilized oleic acid | 对照 Control | 高温灭菌 Moist heat sterilized oleic acid | 过滤除菌 Filtered sterilized oleic acid |
| 主要出峰数 The number of main peaks | 4 | 6 | 6 | 4 | 5 | 5 | 6 | 7 | 8 |
| 总面积 Total area (mAU·min) | 40 841 | 184 009 | 223 336 | 83 725 | 687 430 | 236 126 | 352 088 | 545 365 | 792 093 |
注:
Note: The number of main peaks in
3 讨论
前期研究通过添加不同的脂肪酸和表面活性剂发酵确定了油酸对灵芝三萜的促进作用最佳(Feng et al. 2017),孙冰沁(2017)也优化了油酸促进灵芝液态发酵的工艺。但是以上相关研究的油酸添加浓度过高,缺少添加工艺的系统化研究。本研究详细优化了添加油酸的工艺,在添加少量油酸的情况下大幅度提升菌丝体生物量以及三萜含量。本研究结合单因素实验和响应面实验方法,对油酸的添加方式、添加浓度和添加时间进行工艺优化,得到在第32h添加1.21%的高温灭菌油酸、在第7h添加1.35%的过滤除菌油酸,菌丝体快速增长的同时三萜含量达到最高,分别为42.69mg/g和43.38mg/g。
将响应面最优工艺在不同的发酵规模下进行验证,对不同发酵规模的发酵过程参数进行比较(表8)。发酵所得的最大菌丝体生物量、灵芝三萜得率、菌丝体生产强度以及灵芝三萜生产强度在250mL摇瓶规模下添加过滤除菌油酸高于高温灭菌油酸,在1 000mL摇瓶和3L发酵罐规模下添加油酸高于对照。还原糖消耗速率随着发酵规模的增大逐渐降低,可能与其发酵条件相关;添加油酸后还原糖利用率较低,可能是因为油酸作为辅助碳源参与灵芝液态深层发酵,降低了葡萄糖的利用。
表8 不同培养规模下的灵芝菌丝体发酵参数比较
Table 8
| 参数名称 Parameters | 发酵规模 Fermentation scales | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 250mL摇瓶 250mL shaking flask | 1 000mL摇瓶 1 000mL shaking flask | 3L发酵罐 3L fermenter | |||||||
| 对照 Control | 高温灭菌 Moist heat sterilized oleic acid | 过滤除菌 Filtered sterilized oleic acid | 对照 Control | 高温灭菌 Moist heat sterilized oleic acid | 过滤除菌 Filtered sterilized oleic acid | 对照 Control | 高温灭菌 Moist heat sterilized oleic acid | 过滤除菌 Filtered sterilized oleic acid | |
| 初始还原糖浓度 Initial reducing sugar concentration (g/L) | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| 最终还原糖浓度 Final reducing sugar concentration (g/L) | 5.4 | 11.2 | 11.5 | 3.2 | 12.25 | 13.65 | 15 | 15.4 | 15.5 |
| 发酵时间 Fermentation time (h) | 168 | 168 | 168 | 168 | 168 | 168 | 168 | 168 | 168 |
| 最大菌丝体生物量 Maximum mycelial biomass (g/L) | 10.12 | 14.42 | 20.79 | 7.22 | 13.63 | 14.01 | 7.76 | 11.67 | 13.20 |
| 灵芝三萜得率 Triterpene yield (g/L) | 0.212 | 0.610 | 0.886 | 0.120 | 0.463 | 0.461 | 0.137 | 0.334 | 0.332 |
| 还原糖消耗速率 Reducing sugar consumption rate [g/(L·h)] | 0.087 | 0.052 | 0.051 | 0.100 | 0.046 | 0.038 | 0.030 | 0.027 | 0.027 |
| 菌丝体对还原糖得率 Mycelial yield on reducing sugar (g/g) | 0.693 | 1.639 | 2.446 | 0.430 | 1.759 | 2.206 | 1.552 | 2.537 | 2.933 |
| 灵芝三萜对还原糖得率 Triterpene yield on reducing sugar (×10-2g/g) | 1.449 | 6.933 | 10.421 | 0.715 | 5.971 | 7.254 | 2.740 | 7.261 | 7.378 |
| 菌丝体生产强度 Mycelial productivity [g/(L·h)] | 0.060 | 0.086 | 0.124 | 0.043 | 0.081 | 0.083 | 0.046 | 0.069 | 0.079 |
| 灵芝三萜生产强度 Triterpene productivity [10-3g/(L·h)] | 1.259 | 3.632 | 5.273 | 0.715 | 2.754 | 2.742 | 0.816 | 1.991 | 1.975 |
使用比色法和高效液相色谱(HPLC)法对不同发酵规模的菌丝体三萜含量进行检测,这是目前主要检测灵芝三萜的方法,比色法可以简单、快捷地检测出灵芝三萜含量,但该方法没有特异性,检测结果容易受到干扰物质的影响。HPLC法可以更精准地检测出三萜含量,但所对应的标准品数量大、价格昂贵,检测时间较长(张倩倩和黄青 2018)。结合使用比色法和HPLC法检测菌丝体中的三萜含量,可以充分证明油酸促进菌丝体生物量和三萜含量的提高。
本研究虽然证明了油酸对灵芝液态发酵有促进作用,但是在使用HPLC法检测时,缺少标准品对照,并未确认对何种灵芝三萜有促进作用,这些问题将在后续实验中进行研究讨论。在灵芝液态深层发酵的过程,通过添加油酸可以促进菌丝体的生长和灵芝三萜含量的提高,添加方式的不同导致最终结果产生一定差异,可能是因为经过不同方式处理,油酸自身的营养物质产生变化,也可能是在发酵过程中,不同添加方式的油酸被菌丝体利用能力不同,未来可通过机理方面的研究进行分析讨论。
参考文献
Species clarification of the prize medicinal Ganoderma mushroom “Lingzhi”
DOI:10.1007/s13225-012-0178-5 URL [本文引用: 1]
Biosynthesis and fermentation control of triterpenoids from Ganoderma lingzhi
Species diversity, taxonomy and phylogeny of Polyporaceae (Basidiomycota) in China
DOI:10.1007/s13225-019-00427-4 URL [本文引用: 1]
Notes on the nomenclature of the most widely cultivated Ganoderma species in China
A novel Ganoderma lucidum G0119 fermentation strategy for enhanced triterpenes production by statistical process optimization and addition of oleic acid
DOI:10.1002/elsc.201600071
PMID:32624788
[本文引用: 2]

A novel enhanced triterpenes fermentation production process by G0119 with the addition of oleic acid in the medium has been developed and optimized. All of the six exogenous additives tested were found to exhibit stimulatory effect on mycelial growth and triterpenes biosynthesis by. The results show that oleic acid addition had significant role in promoting triterpenes production. The optimal concentration and time of oleic acid addition were determined to be 30 mL/L and 0 h, respectively. Furthermore, three significant factors influencing triterpenes production were identified as glucose, magnesium sulfate and temperature using the Plackett-Burman design. The optimized conditions by central composite design were 27.83 g/L glucose, 1.32 g/L magnesium sulfate, 26.2°C temperature. The triterpenes fermentation yield with the optimized medium based on actual confirmatory experimental data in 6 L fermentor was 1.076 g/L versus the statistical model predicted value of 1.080 g/L. Our innovatively developed triterpenes fermentation production technology and process has been proven to produce high triterpenes productivity and yield conceivably useful for industrial production.© 2016 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.
A method to improve Ganoderma triterpenes in submerged fermentation of Ganoderma lucidum mycelium
Anti-lung cancer activity through enhancement of immunomodulation and induction of cell apoptosis of total triterpenes extracted from Ganoderma lucidum (Leyss. ex Fr.) Karst
DOI:10.3390/molecules18089966 URL [本文引用: 1]
Antibacterial and antiviral value of the genus Ganoderma P. Karst. species (Aphyllophoromycetideae): a review
DOI:10.1615/InterJMedicMush.v5.i3 URL [本文引用: 1]
Ganoderma triterpenoids exert antiatherogenic effects in mice by alleviating disturbed flow-Induced oxidative stress and inflammation
Research progress of the effect of oils on edible and medicinal mushrooms
The effects of exogenous substances coix seed oil on the liquid fermentation of Ganoderma lucidum triterpenes
Research progress on preparation and pharmacological activities of Ganoderma lucidum triterpenes
Suppression of the inflammatory response by triterpenes isolated from the mushroom Ganoderma lucidum
DOI:10.1016/j.intimp.2009.07.011 URL [本文引用: 1]
Study on fermentation of Ganoderma lucidum mycelium with high triterpenoids’ production based on oleic acid induction and the effect of oleic acid on biosynthesis of key enzymes
Determination of total triterpenoids in fruiting body and spores of Ganoderma lucidum and assessment of their antitumor activity in vitro
Inhibition of tumor cell proliferation by a neutral triterpenoid fraction from Ganoderma lucidum
Antimicrobial effect of the Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (higher Basidiomycetes) and its main compounds
Mushrooms are considered one of the richest sources of natural antibiotics, and various species of them inhibit the growth of a wide diversity of microorganisms. Ganoderma lucidum, a well-known medicinal mushroom. has many pharmacological and biological activities including an antimicrobial effect, although few studies have investigated the antibacterial and antifungal effects of its purified compounds. The chemical structure of the purified compounds from the hexane fraction was elucidated as ergosta-7,22-dien-3β-yl acetate, ergosta-5,7,22-trien-3β-yl acetate (isopyrocalciferol acetate), ergosta-7,22-dien-3-one, ergosta-7,22-dien-3β-ol, and ergosta-5,7,22-trien-3β-ol (ergostrol). In addition, the structure of ganodermadiol was demonstrated after purification from the chloroform fraction. The fractions inhibited Gram-positive bacteria and yeast, with minimum inhibitory concentration values of 6.25 mg/mL, but were ineffective against Gram-negative bacteria in the tested concentrations. The results were comparable for isolated compounds, whereas the mixture of ergosta-7,22-dien-3β-yl acetate and isopyrocalciferol acetate was weakly effective against Escherichia coli (minimum inhibitory concentration, 10 mg/mL). It could be assumed that the antimicrobial effect of crude fractions is the consequence of mixing triterpenoid and steroid compounds.
Effects of triterpenoids from Ganoderma lucidum (Leyss. ex Fr.) Karst. on three different experimental liver injury models in mice
A Review on the effects of exogenous additives on submerged fermentation of edible and medicinal fungi
Resource diversity of Chinese macrofungi: edible, medicinal and poisonous species
DOI:10.1007/s13225-019-00432-7 URL [本文引用: 1]
Effect of fatty acids on the mycelial growth and polysaccharide formation by Ganoderma lucidum in shake flask cultures
PMID:10899556

Fatty acids were added into the media to investigate their effects on the mycelial growth and polysaccharide formation by Ganoderma lucidum. The experiments were carried out in freely suspended cultures or immobilized cultures using shake flasks. The results indicate that the extent of stimulation or inhibition were associated with the types and levels of fatty acids. Oleic acid at the level of 0.15 g/100 ml led to a significant increase in cell concentration from 0.20 to 0.46 g/100 ml in a suspended culture and palmitic acid was of great advantage to polysaccharide production. In contrast, linoleic acid (0.1 g/100 ml) drastically suppressed both mycelial growth and polysaccharide formation. In immobilized cultures with fatty acids, the stimulation of mycelial growth remained the same level, but the enhancement of polysaccharide production became less. In addition, the growth of G. lucidum in the pattern of immobilization might be beneficial to the production of mycelia and polysaccharide.
Determination of triterpenoids in Ganoderma lingzhi spore powder by HPLC
Effect of fatty acid supplementation on mycelium biomass, polysaccharide and triterpene production by Ganoderma lucidum grown in submerged culture
Experimental research on lowering the serum lipid effect of Ganoderma lucidum
Effects of complex organic nitrogen source on triterpene production by Ganoderma lingzhi based on liquid submerged fermentation
Revised method for determining Ganoderma lingzhi terpenoids by UV-Vis spectrophotometry based on colorimetric vanillin perchloric acid reaction
Study on the optimization of culture medium of Ganoderma fermentation before extraction of ganoderic acid